RQM+ Medical Device and In Vitro Diagnostic Blog
Posts By: Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
Subscribe to our blog
Sorry, no listings found for that Search. Try changing your fiter and search again.
-

Summary of FDA Regulatory Changes for Digital Health Devices in Q3 2022
on 6 December 2022 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
Digital health devices are no longer new on the scene, but the regulatory landscape continues to evolve. As predicted in our blog post earlier this year, the latest development is new U.S. Food and Drug Administration (FDA) guidance documents for digital health devices that were...
Read More -

How the FDA Encourages Innovation in Pediatric Medical Devices and Best Practices for Submissions
on 8 November 2022 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
As an industry, medical device manufacturers and regulators have an obligation to ensure that children are not left behind when it comes to the innovation and development of products to treat childhood conditions and diseases. The reality is that the innovation of pediatric...
Read More -

Lessons Learned From Using the FDA Customer Collaboration Portal (CCP) for eCopy and eSTAR
on 3 October 2022 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
As part of its commitment to shift to electronic submission, the Center for Devices and Radiological Health (CDRH) at the Food and Drug Administration (FDA) launched the Customer Collaboration Portal (CCP) in July 2022. The CCP allows medical device manufacturers to...
Read More -

Med Device Monday - Luminopia One, A Digital Therapeutic to Help Treat Amblyopia
on 25 October 2021 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

FDA Friday - Anike Freeman, M.Eng., PMP
on 8 October 2021 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
This #FDAFriday series consists of mini-interviews with former FDA regulators. Our goals are twofold: (1) help students and professionals interested in Regulatory Affairs see what career paths are possible, and (2) talk about some of the various roles at FDA to demonstrate the...
Read More -
Med Device Monday - The Sonalleve MR-HIFU System Approved to Treat Osteoid Osteomas
on 25 May 2021 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
Med Device Monday - Novel Device to Reduce Snoring and Mild Obstructive Sleep Apnea
on 26 April 2021 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

FDA Friday- Misleading Registration Certificates: Sorting through Company Claims
on 19 March 2021 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
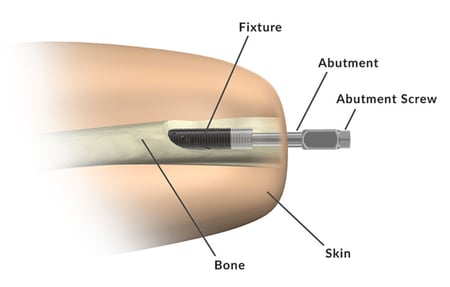
Med Device Monday - FDA Approves Prosthetic Implant for Above-the-Knee Amputations
on 22 February 2021 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
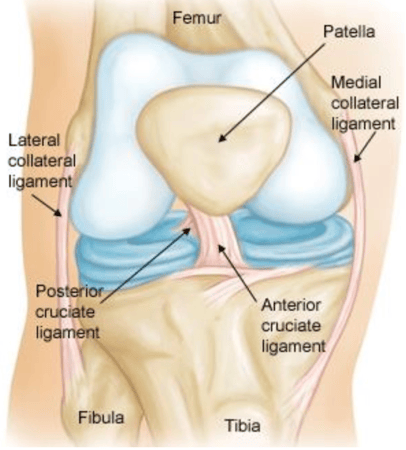
Med Device Monday - New Implant to Repair Torn ACL
on 4 January 2021 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

FDA Friday - Interview with Dr. Seth Carmody
on 30 October 2020 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
Med Device Monday - Cybersecure Medical Devices
on 26 October 2020 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

FDA Friday - Cybersecurity Awareness Month at FDA!
on 23 October 2020 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

Med Device Monday: The iStent inject Device for Glaucoma Patients
on 27 January 2020 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

Med Device Monday – FDA grants a new device that can detect HIV-1 Drug Resistance Mutations
on 9 December 2019 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
Med Device Monday: TransMedics OCS™ Lung System for Lung Preservation
on 28 October 2019 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
Med Device Monday - XVIVO Perfusion System for Storing Human Organs Before Transplantation
on 30 September 2019 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

Wednesday Wisdom: Article on Regulating Software as a Medical Device (SaMD)
on 5 June 2019 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
Med Device Monday: The Fluobeam 800 and the PTeye System for Parathyroid Tissue Detection
on 7 January 2019 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
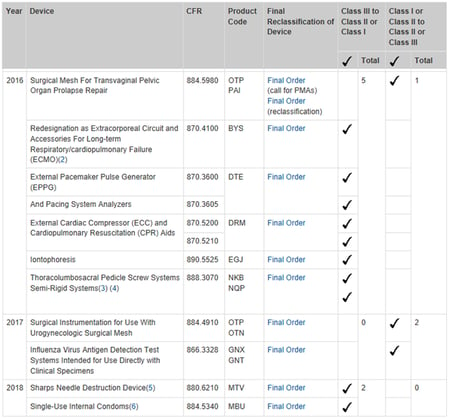
FDA Friday: Reclassification
on 30 November 2018 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

Med Device Monday: Aethlon's Hemopurifier
on 29 October 2018 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

FDA Friday: Updates to our Previous De Novo Pathway Blog
on 14 September 2018 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
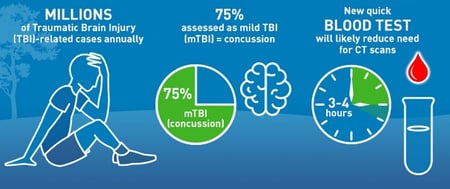
Med Device Monday: Diagnostic for Detection of Brain Trauma
on 2 April 2018 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
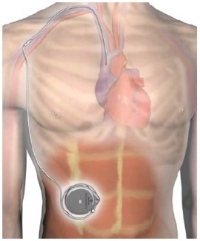
Med Device Monday: Implantable Continuous Pump for Remodulin
on 6 March 2018 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

FDA Friday - Michael Nilo, MS
on 12 January 2018 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
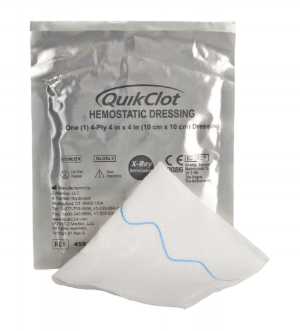
Med Device Monday: Nonabsorbent Dressing
on 31 July 2017 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
Med Device Monday: Class II Device Types Exempt from Premarket Notification
on 18 July 2017 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

Medical Device Monday: Tongue Depressors
on 4 July 2017 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

Med Device Monday: Bone Mills
on 30 May 2017 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

What's Changed with FDA User Fees?
on 16 May 2017 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
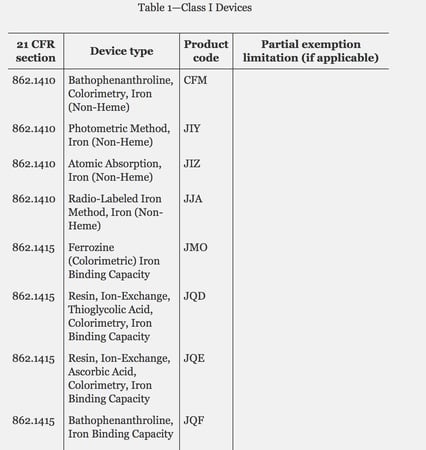
Med Device Monday: Class I Device Types Exempt from Premarket Notification
on 17 April 2017 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
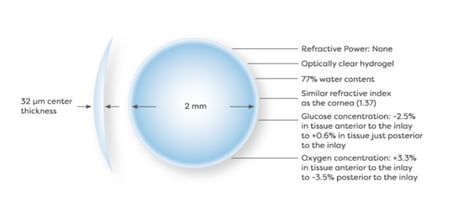
Med Device Monday: Raindrop Vision Inlay
on 11 April 2017 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

Med Device Monday: C-Section Retractor
on 28 March 2017 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

Med Device Monday: Restless Leg Relaxer
on 24 January 2017 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
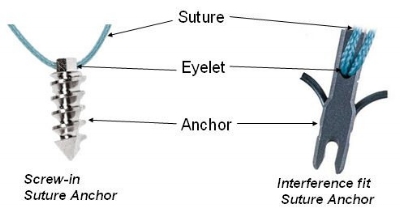
Med Device Monday: Bone Anchors
on 10 January 2017 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
Med Device Monday: Briteseed and SafeSnips
on 3 October 2016 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
Med Device Monday: Prosthetic Face
on 16 August 2016 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
The de novo Pathway: Is It Right for My Device?
on 10 June 2016 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -
Wellness Devices vs. Medical Devices
on 3 June 2016 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

How I Broke into Regulatory Affairs, and How You Can, Too
on 13 May 2016 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More
GLOBAL BOTTOM CTA INSTRUCTIONS:
To display custom copy instead of global copy in this section, please go to Show Global Content for Bottom CTA? toggle in the "Contents" tab to the left, toggle it off, save, and then REFRESH the page editor, the custom text will then show up and ready to be edited.
Turning the global content back on will be the same process, go to the toggle and toggle it back on, save and refresh!


