In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of current and former FDA reviewers, scientists, engineers and regulatory and quality experts, and adds additional expertise with FDA submissions. The author of this post is a member of this team, which has done significant work with novel and/or high-risk devices focusing on pre-submissions, 510(k)s, IDEs, PMAs, De Novos, Breakthrough Designation Requests and Safer Technology Program Requests.
Do you or a loved one boil turnips? Mow hay? Rake up the coals? Saw logs? Well, there’s a device for that! More specifically, FDA granting of a new device may help people reduce their snoring and reduce mild obstructive sleep apnea.
Sleep is so important, and yet it is very often one of the first things we sacrifice when life gets busy and we need a few more hours in the day. Humans require sleep because it is during sleep that our bodies have the opportunity to restore the immune, nervous, skeletal, and muscular systems, which in turn support our mood, memory, physical well-being and cognitive function. While some of us would get more and/or better sleep by practicing a better bedtime routine, many suffer from sleep disorders that can cause the person (and often the person sleeping next to them) to not get enough or sufficient quality sleep. For example, obstructive sleep apnea is a condition in which major pauses in breathing occur during sleep. These pauses disrupt the normal progression of sleep and often lead to other health problems. While perhaps less dangerous, even snoring can cause sleep deprivation in snorers and those around them, and has been linked to health conditions such as cardiovascular disease, obesity, and mental illness.
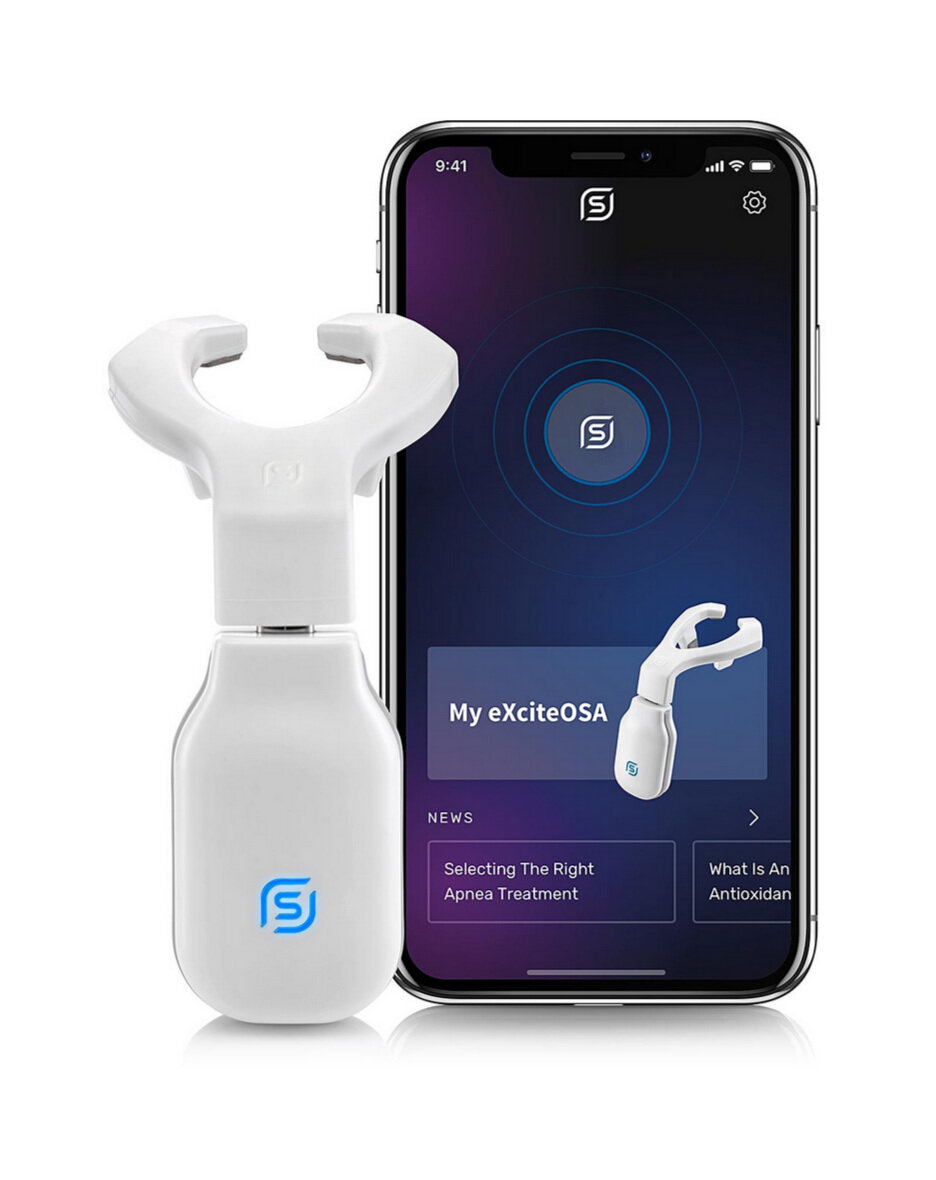
In early February, 2021, the U.S. FDA authorized marketing of a new prescription only device intended to reduce snoring and mild obstructive sleep apnea. Unlike devices used while patients sleep, such as dental appliances and continuous positive airway pressure (CPAP) machines, the new device is the first device intended to be used while the wearer is awake. The device, known as the eXciteOSA, is a removable tongue muscle stimulation device that delivers neuromuscular stimulation to the tongue to improve tongue muscle function, which in time can help prevent the tongue from collapsing backwards and obstructing the airway during sleep.
The eXciteOSA device works by delivering electrical muscle stimulation through a mouthpiece that sits around the tongue. The eXciteOSA mouthpiece has four electrodes, two located above the tongue and two located below the tongue. The device provides electrical muscle stimulation action in sessions that consist of a series of electrical pulses with rest periods in between. It is used for 20 minutes once a day during a wakeful state, for a period of 6‐weeks, and once a week thereafter. In a study of 48 patients with snoring and mild sleep apnea, use of the eXciteOSA mouthpiece reduced the number of episodes of shallow or ceased breathing experienced by each patient by almost half.
FDA granted the marketing authorization to Signifier Medical Technologies, LLC, after reviewing the device through the De Novo premarket review pathway. This means that subsequent devices of the same type with the same intended use may go through FDA’s 510(k) premarket notification process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a predicate device. When met, the special controls, along with general controls, provide reasonable assurance of safety and effectiveness for devices of this type.
The eXciteOSA offers a new option for the thousands of individuals who experience snoring or mild sleep apnea, and the recent news is definitely music to our ears [cue Simon and Garfunkel’s “The Sound of Silence.”
Further Reading


