We Don’t Make MedTech
We Make MedTech Happen
RQM+ is The MedTech CRO – a full-service partner adding expertise from concept to post-market.

We don’t build medical devices.
We make them market-ready.
We don’t invent life-changing diagnostics.
We make sure they meet global standards.
We don’t create combination products.
We make them accessible to patients.
As The MedTech CRO, RQM+ makes MedTech happen by accelerating innovation from concept to patient impact. Our tailored solutions throughout the product life cycle deliver regulatory and quality expertise, FDA-recognized laboratory services, clinical trials, and reimbursement strategies across device types and therapeutic areas to bring life-changing technologies to patients — faster, safer, better.
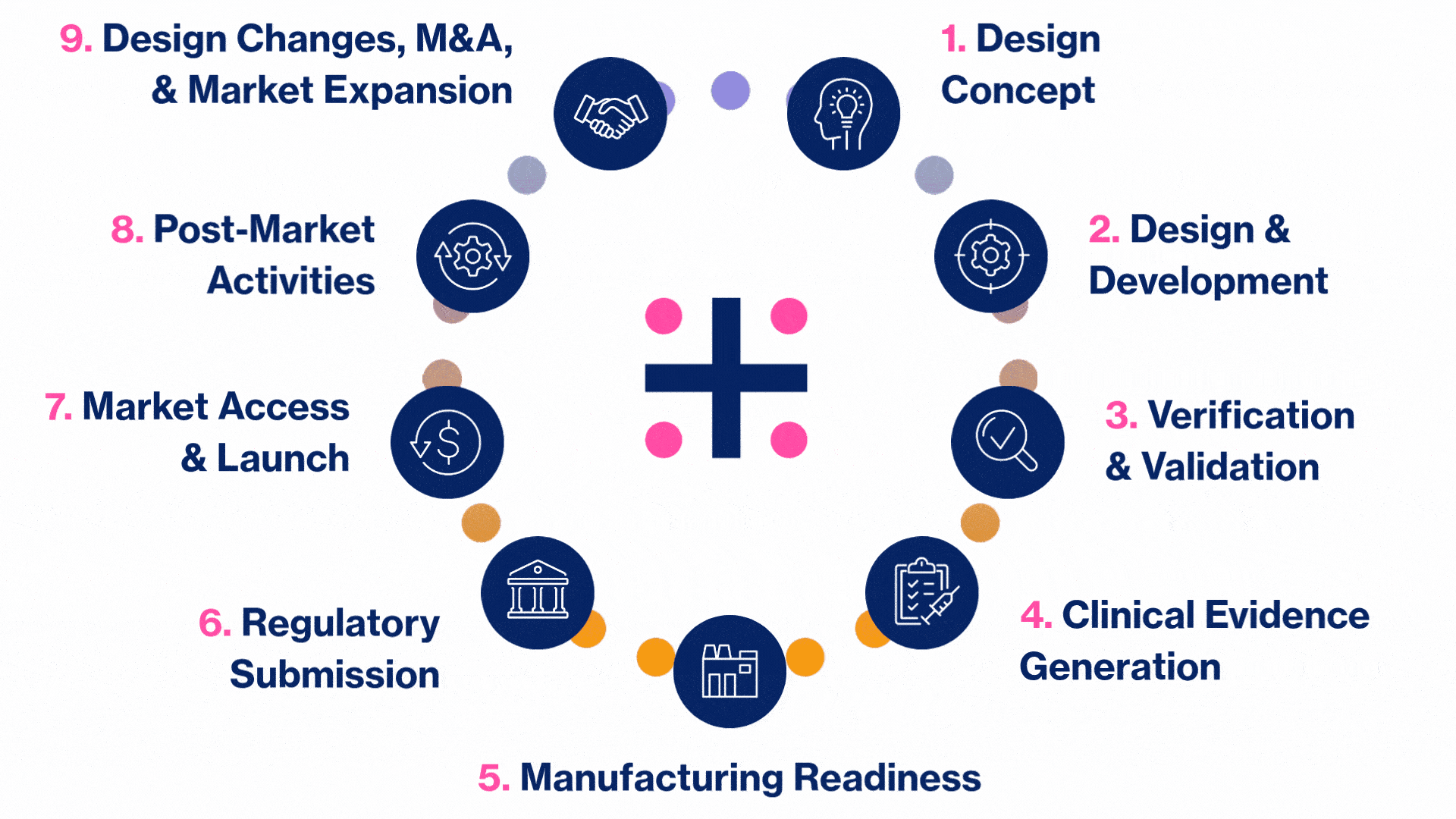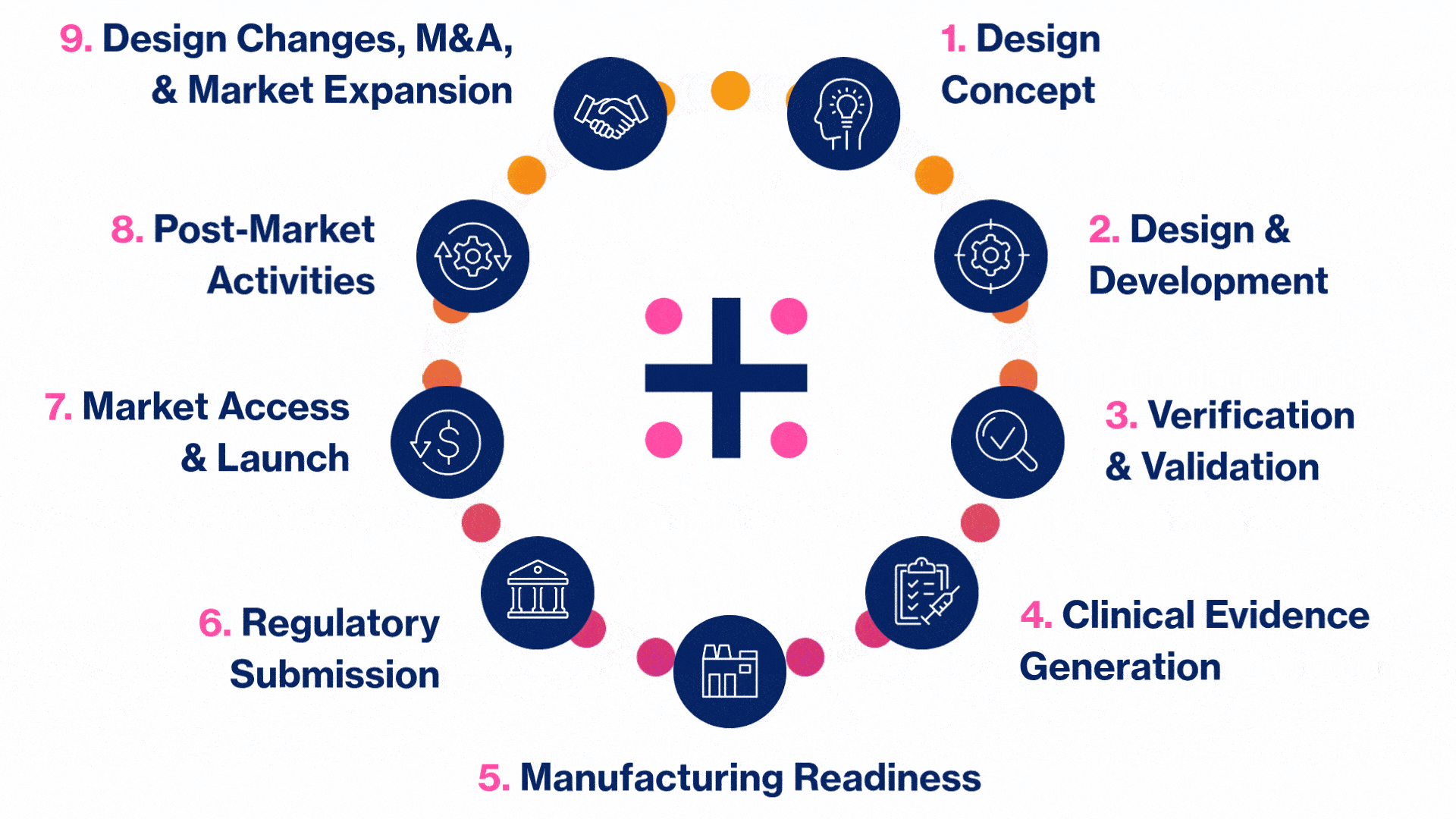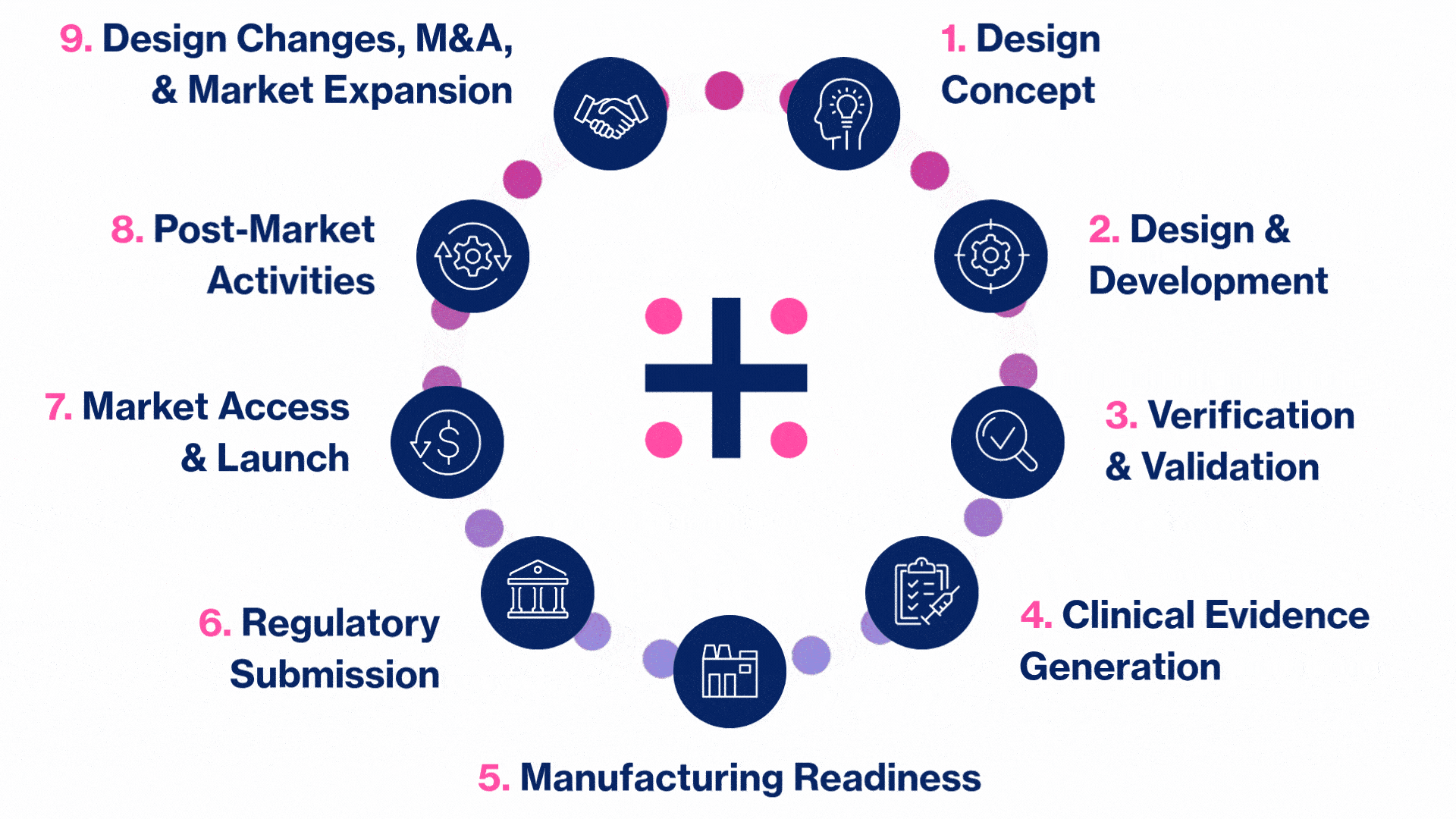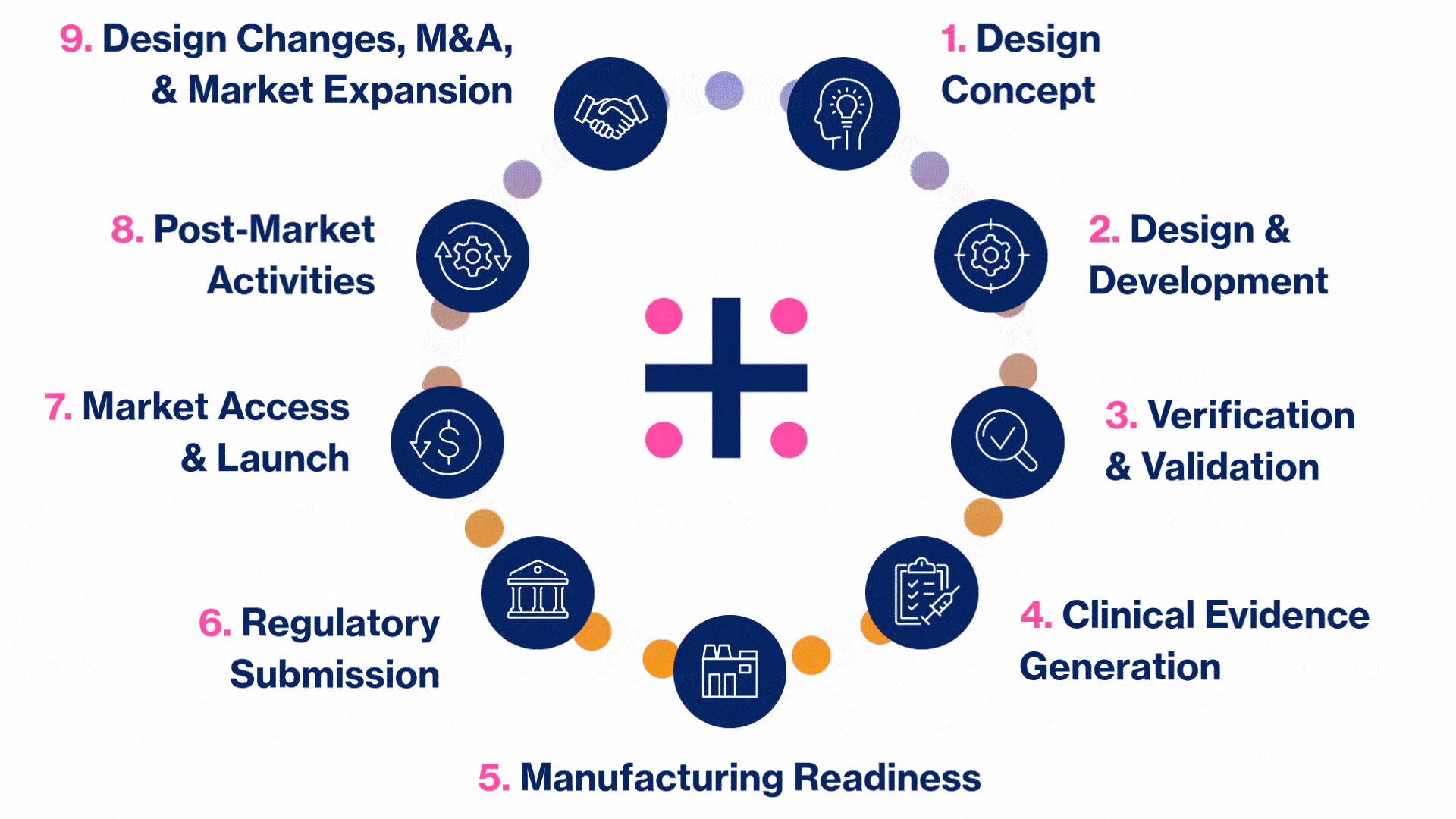
MedTech Solutions for Every Stage of Your Product’s Life Cycle
We provide tailored expertise across four core service and diverse therapeutic areas to advance your MedTech product from concept to market.
Why RQM+
Guiding your MedTech journey with faster solutions, fewer obstacles, and complete support from start to finish.
-
Faster Time to Market
Reduce regulatory delays with precise, experienced guidance.
-
Lower Risk
Identify and resolve challenges before they escalate.
-
Comprehensive Support
From concept to post-market, we ensure every step is seamless.
Flexible Partnering Solutions

Outsourcing
Customizable and scalable outsourcing scoped for entire projects, specific functions, or defined parts of a function. Leverage RQM+ expertise and flexibility while your teams stay focused on core competencies, corporate goals, and innovation. Partner with us.

Consulting
Targeted, outcome-focused expertise to solve defined MedTech challenges. RQM+
consultants step in with practical, actionable guidance and hands-on execution to drive timely resolution. Partner with us.

Staff Augmentation
Fill skill or capacity gaps with MedTech talent from RQM+. We provide on-demand
professionals, functional service support, fast deployment for tight timelines, and
contract-to-hire options that integrate seamlessly with your team. Match with a contractor.
Delivering Real Results
Real results that demonstrate our expertise and commitment to your MedTech success:
-
40+ Years
of ExpertiseDelivering regulatory and quality (RA/QA) consulting excellence.
-
19 of the Top 20 Medical
Device CompaniesTrusted by industry leaders worldwide.
-
7 of the Top 10 IVD
CompaniesChosen by innovators for their most critical projects.
-
50% Efficiency
GainAchieved through our proprietary technology platforms.
-
Gold Standard Lab & Material Science Through Jordi Labs
Industry-leading materials testing and chemical characterization recognized by the FDA.
-
Global
ReachSuccessful regulatory submissions across the U.S., Europe, and the U.K.
Hear From Our Clients
Trusted by industry leaders to simplify the complex and help bring life-changing innovations to market.