Complete Managed Outsourcing
Trust RQM+ to handle every aspect of independent regulatory and quality functions from supplier audits to post-market surveillance reporting and beyond.
“Without a doubt, RQM+ is our number one partner!”
– Multinational medical device manufacturer
Are you challenged to manage your organization's short, mid, and long term goals? Is your limited capacity continuing to plague your ability to execute?
Regardless of need, we can bring 30+ years of leadership and delivery success.
RQM+ can be your partner.
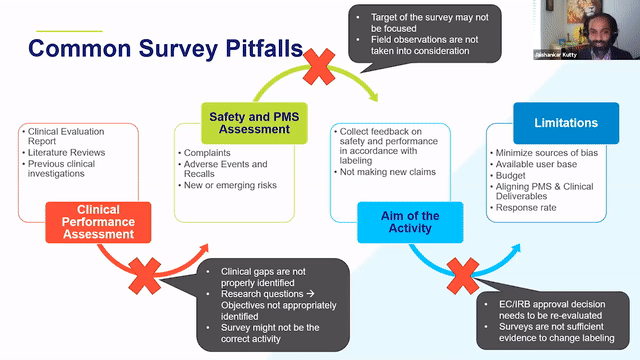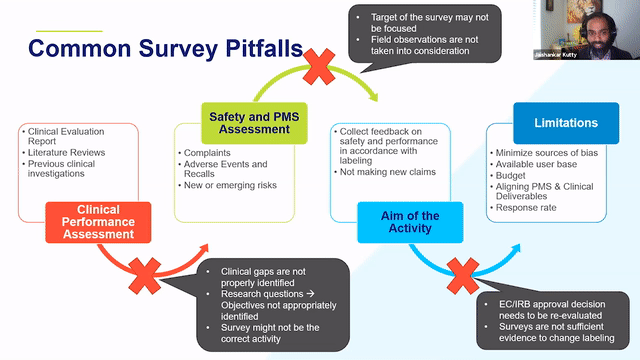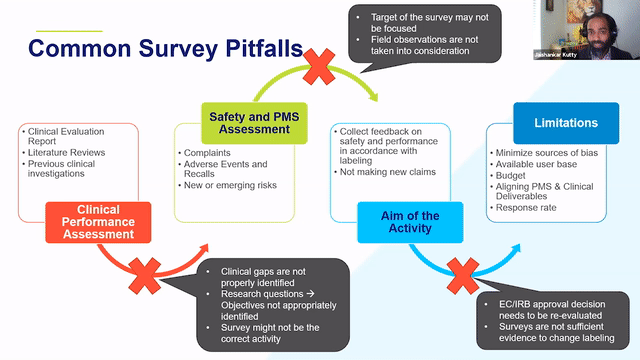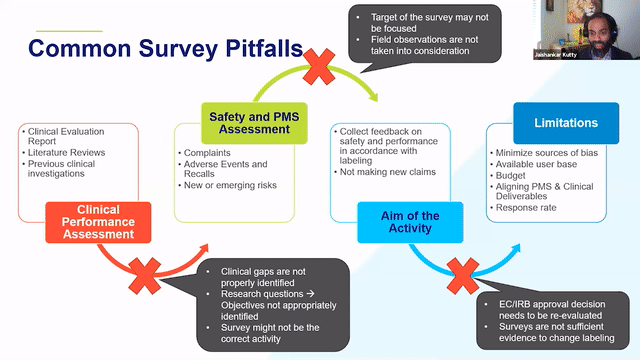
How RQM+ Adds Value
-

Transformative Solutions Make Your Business Better
Even when focused on a specific function, we always look at the big picture and prioritize your business goals. We deliver solutions that not only meet the specific objectives of the task at hand but also optimize processes and improve efficiency.
-

Immediately Add Expertise to Your Team
Trusting a partner to take on key regulatory and quality functions can be a concern, especially for critical tasks that require specialized expertise.
RQM+ holds strong experience in providing services on a managed outsource basis, partnering with our clients to achieve business objectives.
-

Redirect Internal Resources
Although certain tasks—such as complaint management, retrospective PMCF/PMPF studies, and CERs/PERs—are necessary for maintaining compliance, they do not necessarily contribute to your larger business goals. By allowing RQM+ to manage these types of functions, you can free up valuable internal resources to focus on the future.
With RQM+ managed outsourcing, you’re not giving up control—you’re gaining expertise.
We understand why manufacturers are sometimes reluctant to hand over the reins. Every aspect of regulatory and quality compliance is important and cannot be undervalued. As medical device regulations get more stringent, we foresee a shift to more outsourcing, just as pharmacovigilance is a function that is primarily outsourced in the pharmaceutical industry. RQM+ has the talent in-house, and we are here when you need us.
Industry Expertise
No matter how many products you sell or what class they are, the experienced team at RQM+ has done it all. With former regulators and notified body leadership on staff, we know how to help you maintain compliance—even with the new EU MDR and IVDR regulations.
Medical Device Regulatory Affairs in the US
From leading pre-market strategy to ensuring post-market compliance and all of the regulatory submissions in between, RQM+ supports regulatory affairs teams throughout the product life cycle. With former FDA regulators on our team, we know what reviewers look for. This allows us to draft submissions that generate fewer questions and helps get your products to market faster.
Retrospective PMCF/PMPF Studies
The new EU regulations require ongoing post-market clinical and performance studies, and many manufacturers are struggling to figure out how they will execute this mandate. Clinical and technical expertise is required to design meaningful studies, deploy them, and compile the data so they will be acceptable to notified bodies in your PMCF report. RQM+ has the experience and expertise (including former NB leadership), client-validated processes, and tools to conduct studies effectively with a nearly turnkey approach.
Clinical Regulatory Affairs
The work isn’t done after a product has been approved and on the market. RQM+ can handle clinical regulatory affairs for entire product lines or therapeutic categories so you can be confident that your clinical and performance evaluation reports (CERs and PERs), periodic safety update reports (PSURs), and other required documentation are always submitted on time to maintain compliance with minimal burden on your team.

RQM+ Live!
Overcoming Challenges with Integrating PMS, CERs/PERs and Risk Management under EU MDR and IVDR

Device Advice Podcast
Creating Performance Evaluation Plans and Reports under the IVDR
FAQ
Can you have a CER with multiple products, some of which are under the MDR and some that will remain under the MDD until a later date?
Auditing
Audits are extremely time consuming, especially if you operate in multiple markets and have suppliers around the world. Shifting attention to audits can have a negative impact on other important quality management tasks, especially if a significant portion of your audit team are resources that belong to other departments. RQM+ can provide dedicated global auditing services that will improve the efficiency and effectiveness of your audit program, while allowing your team to concentrate on essential processes.
Complaint Management
The organizational expertise of a manufacturer is often focused on product development and proactive risk management in an effort to avoid complaints. Unfortunately, though, complaints will happen—and typically, more than expected. Although complaint handling is a critical function for post-market surveillance, it can be a burden on internal teams and detract from the work required to meet business objectives. RQM+ can handle it all for you and ensure that the complaint handling process integrates seamlessly with other post-market surveillance systems.
With complete managed outsourcing, you get a strategic partner that works with the best interest of your business in mind and seasoned experts who deliver added value.
01 Dedicated Team
Our solutions provide a dedicated team of resources that handle more than just the technical tasks—you’ll have project management, resource management, and technical leadership like you’ve never had before.
02 Single-Vendor Solution
With RQM+ managed outsourcing, we handle everything for you so your team has a single point of contact and only one partner with specific deliverables.
03 Transformative Solutions
We are laser-focused on providing quality service that exceeds expectations and improves your business—and we have the experience to deliver on this promise.
04 Industry Expertise
RQM+ is dedicated to the medical device and diagnostic industries, and has expert resources across all clinical specialties and device classes to support all of your products.
Featured Leader

Eric Pauls
Chief Customer Officer
Eric has spent the majority of his career on the customer side, and brings a powerful perspective to RQM+ by understanding client needs and how to tailor solutions that drive measurable results. Eric is responsible for client relationships and growth.The idea of outsourcing is sometimes met with reluctance because it feels like you are losing control over important functions with reliance on a third party. Not with RQM+. We integrate fully with your team and act as a strategic partner to add value to your business through transformative solutions.

GLOBAL BOTTOM CTA INSTRUCTIONS:
To display custom copy instead of global copy in this section, please go to Show Global Content for Bottom CTA? toggle in the "Contents" tab to the left, toggle it off, save, and then REFRESH the page editor, the custom text will then show up and ready to be edited.
Turning the global content back on will be the same process, go to the toggle and toggle it back on, save and refresh!