RQM+ Medical Device and In Vitro Diagnostic Blog
Topic: IVD
Subscribe to our blog
Sorry, no listings found for that Search. Try changing your fiter and search again.
-

Global Perspectives: Comparing Regulations for Point of Care Tests in the U.S. and EU
on 3 October 2024 | By Margot Borgel, Ph.D., Director, IVD Global Regulatory Affairs, RQM+
Point of Care tests (POCT) is a term that is widely used to describe IVDs that are used at the same location as the patient, as opposed to an IVD that is used within a laboratory. This can include a wide range of locations including doctor's offices, clinics, patient bedside,...
Read More -

Impact of FDA's Final LDT Rule: Industry Concerns and Compliance
on 29 July 2024 | By Margot Borgel, Ph.D., Director, IVD Global Regulatory Affairs, RQM+
FDA released the final rule on laboratory developed tests (LDTs) on May 6, 2024. This rule brings most LDTs under the purview of FDA through the medical device regulation by including them within the definition for in vitro diagnostics (IVDs).
Read More -

Medical Device Software: Top Regulatory Submission/File Deficiencies and Requests for Additional Info From Both FDA and Notified Bodies
on 29 November 2022 | By Kevin Go, MBA, RAC, CQA, RQM+ Senior Principal
Medical devices that fall into the category of software as a medical device (SaMD) are on the rise as technology continues to evolve. Although these products have proven to be highly beneficial to patients and end users, manufacturers must overcome many regulatory challenges.
Read More -

Just Released: MDCG Guidance 2022-3 Verification of Manufactured Class D IVDs by Notified Bodies
on 17 February 2022 | By Heike Moehlig-Zuttermeister, PhD, RQM+ VP Intelligence and Innovation, IVD
The MDCG guidance 2022-3 Verification of manufactured class D IVDs by notified bodies was issued earlier this week on the 15th of February 2022. This guidance is important for manufacturers because it provides a detailed specification of the requirements for the testing plan and...
Read More -

EU Update: Expert Panel Views for SARS-CoV-2 Devices
on 2 February 2022 | By Heike Moehlig-Zuttermeister, PhD, RQM+ VP Intelligence and Innovation, IVD
The first three Performance Evaluation Consultation Procedure (PECP) views have been published for a professional SARS-CoV-2 immunoassay and a professional nucleic acid testing (NAT) assay as well as a near-patient testing NAT SARS-CoV-2 product. Considering the actual...
Read More -

RQM+ Executive Video Briefing: IVDR New Transition Timelines
on 17 January 2022 | By RQM+ Subject Matter Experts
While we are happy and relieved for the IVD industry to have an extension to the IVDR transition period, we’re concerned that the announcement may result in a misperception that there is plenty of time to get the work done.
Read More -

The IVDR Performance Evaluation Report
on 21 December 2021 | By RQM+ Subject Matter Experts
Under the EU In Vitro Diagnostic Medical Devices Regulation 2017/746 (IVDR), every IVD must have a Performance Evaluation Report (PER). PERs consist of three pillars: scientific validity, analytical performance and clinical performance. Collating data to satisfactorily address...
Read More -

Clinical Evidence Requirements Under IVDR
on 16 December 2021 | By RQM+ Subject Matter Experts
The next key regulatory milestone for manufacturers with products on the European market is the EU In Vitro Diagnostic Medical Devices Regulation 2017/746 (IVDR). A critical stage of IVDR planning is for manufacturers to assess whether they have enough clinical and performance...
Read More -

Canada Regulations News for Medical Devices and IVDs
on 3 December 2021 | By Nancy Morrison, RQM+
In case you missed it, the Medical Device Regulations in Canada quietly got updated in December 2020. The new regulation intends to boost post-market safety and requires manufacturers to submit a summary report, which is similar to the post-market Periodic Benefit-Risk...
Read More -

Facts & FAQs: European Commission Proposal for Amendment of the IVDR (EU) 2017/746
on 19 October 2021 | By RQM+ Subject Matter Experts
Background The COVID-19 pandemic has led to unprecedented challenges for the In Vitro Diagnostic Regulation (IVDR) implementation impacting both manufacturers and key European Union (EU) infrastructure. On one hand, manufacturers were faced with staff shortages impacting their...
Read More -
EU Update: SARS-CoV-2 In Vitro Diagnostic Medical Device Performance Evaluation MDCG Guidance Published
on 13 September 2021 | By Ashley (Ash) Clark, MS, RAC, RQM+
A new guidance document (MDCG 2020-21) has been published on the 3rd of August by the Medical Device Coordination Group (MDCG) pertaining to performance evaluation of SARS-CoV-2 in vitro diagnostic medical devices. This document provides extensive details on what is expected for...
Read More -
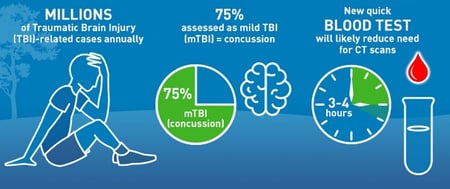
Med Device Monday: Diagnostic for Detection of Brain Trauma
on 2 April 2018 | By Allison Komiyama, Ph.D., RAC, RQM+ Vice President, MedTech Innovations
In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of...
Read More -

R&Q's Can't-Miss Education Event of the Year: An Afternoon Training Workshop at BIOMEDevice in Boston, April 13th
on 8 March 2016 | By Stephen Biernacki
Those in the northeast, get ready! Not only will R&Q be exhibiting at this year's BIOMEDevice show (booth 640) and sponsoring MassMEDIC's Annual Conference, but we'll be presenting an afternoon training workshop (three separate sessions) free to attendees of the BIOMEDevice show...
Read More -
Mdd | Eu | Europe | Medicaldevices | Medical Device | European Union | Medical Device Directives | Ivd | Medicaldevice | Active Implantable Medical Device
Overview of New Medical Device Regulations in Europe
on 26 October 2012 | By Ryan Kasun
This week my inbox was flooded by notifications of a great blog post from the Emergo group, outlining the changes set to occur to the medical device regulations in Europe. Changes such as:
Read More
GLOBAL BOTTOM CTA INSTRUCTIONS:
To display custom copy instead of global copy in this section, please go to Show Global Content for Bottom CTA? toggle in the "Contents" tab to the left, toggle it off, save, and then REFRESH the page editor, the custom text will then show up and ready to be edited.
Turning the global content back on will be the same process, go to the toggle and toggle it back on, save and refresh!



