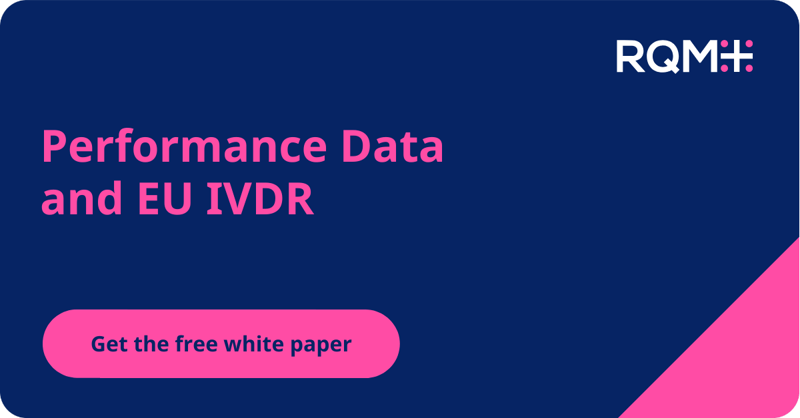
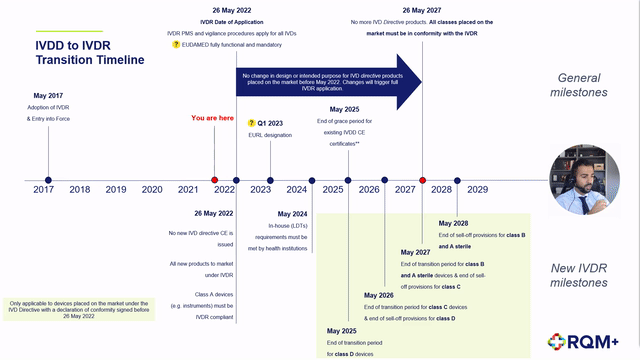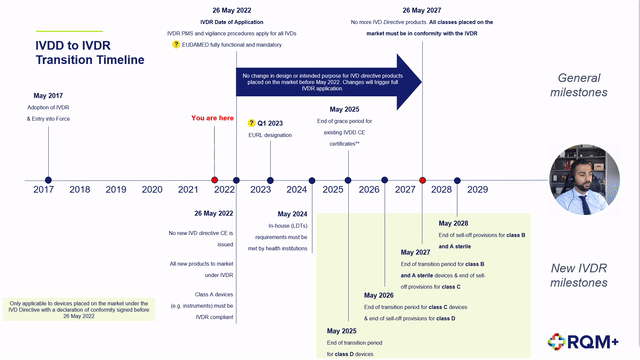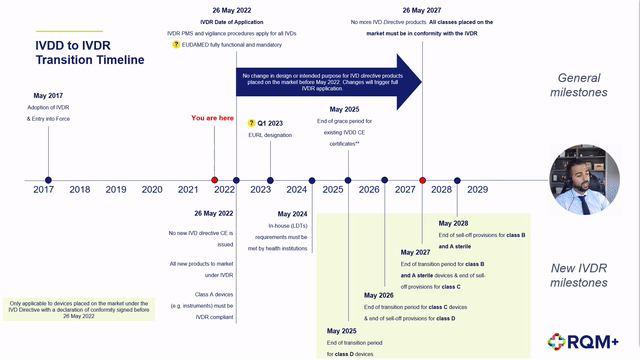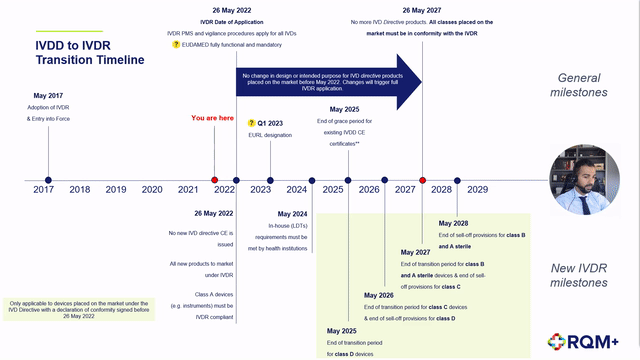
While we are happy and relieved for the IVD industry to have an extension to the IVDR transition period, we’re concerned that the announcement may result in a misperception that there is plenty of time to get the work done.
In this technical video, we dissect the regulation and show visually what a manufacturer needs to accomplish on the revised timeline, including:
➕ Description of the extension to the IVDR transition period,
➕ Detailed description of the impact to each device classification as well as in-house devices,
➕ Proposed step-by-step product journey on the revised timeline including expected challenges,
➕ Predicted notified body product review timelines and concerns, and
➕ Post CE certification work to plan for.
Watch the video below for everything you need to know in less than 30 minutes.
Looking for related content? Download our white paper, “Performance Data and EU IVDR”.