In December 2021, RQM+ acquired AcKnowledge Regulatory Strategies (AcKnowledge RS), a San Diego-based firm specializing in regulatory affairs consulting for the medical device and IVD industry. The integration of this impressive team enhances the extensive RQM+ network of current and former FDA reviewers, scientists, engineers and regulatory and quality experts, and adds additional expertise with FDA submissions. The author of this post is a member of this team, which has done significant work with novel and/or high-risk devices focusing on pre-submissions, 510(k)s, IDEs, PMAs, De Novos, Breakthrough Designation Requests and Safer Technology Program Requests.
Another week, another new medical device here to make a difference! We were delighted to see the FDA news release in December announcing the first implant system marketed in the U.S. for adults who have above-the-knee amputations. There are a number of reasons why someone would need to have their leg removed above the knee joint, including trauma, infection, cancerous tumor, or insufficient blood flow.
As stated by Capt. Raquel Peat, Ph.D., MP.H., USPHS, director of the FDA’s Center for Devices and Radiological Health’s Office of Orthopedic Devices, “approval of the OPRA Implant System expands options for prostheses for individuals who have had above-the-knee amputations, and can help those who have had or may have problems with rehabilitation and have not been able to benefit from available socket prostheses.”
The OPRA Implant system is manufactured by Integrum AB in Mölndal, Sweden, and has actually been on the U.S. market under a humanitarian device exemption (HDE) since 2015. However, FDA recently completed its review of the OPRA Implant System under the Premarket Approval (PMA) pathway. The Agency determined that the PMA application contained sufficient valid scientific evidence to provide reasonable assurance that the device is safe and effective for its intended use.
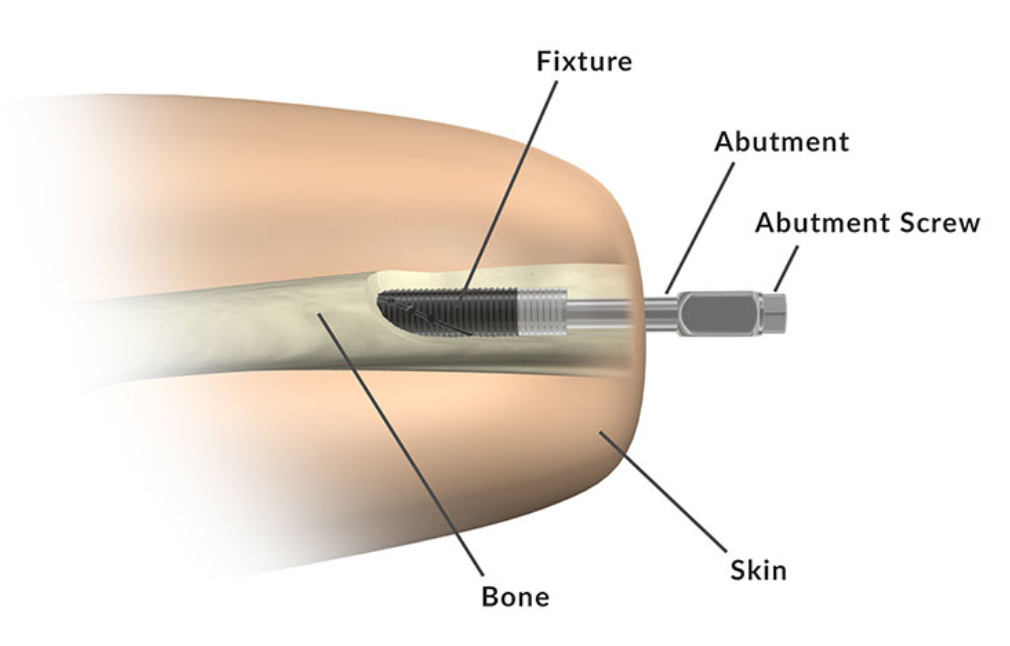
According to the Integrum AB website, “The OPRA Implant System is a bone anchored prostheses system based on osseointegration, where the prostheses are directly attached to the bone.” The system consists of the anchoring element and a skin penetrating connection that is secured with a screw. The device is installed during two surgical procedures. In the first procedure, the cylinder-shaped anchoring element, also known as the fixture, is implanted into the central canal of the remaining thigh bone. Approximately six months later, after tissue has grown to anchor the fixture and the skin tissue has healed, a second surgery is performed to attach additional device components of the OPRA Implant System to the fixture from the previous surgery. The OPRA Implant System extends through the skin at the bottom of the patient’s residual limb and connects to the prosthesis.
After the second surgery, the patient works with a trained physical therapist to gradually place weight on the OPRA Implant System using a training prosthesis. Patients require about six months of training and rehabilitation after the second surgery, before being fitted with their own customized prosthesis.
A conventional leg prosthesis uses a specially-fitted, cup-like shell called a socket that fits over the remaining portion of the patient’s leg to secure the device to the leg. Some patients may not have a long enough residual limb to properly fit a socket prosthesis or may have other conditions, such as scarring, pain, recurrent skin infections or fluctuations in the shape of the residual limb that prevent them from being able to use a prosthesis with a socket. The OPRA Implant System is surgically anchored and integrated into the patient’s remaining thigh bone to allow connection to an external prosthetic limb.
While the clinical study performed to evaluate the safety and effectiveness of the device was not without adverse events, the OPRA Implant System provides patients and their healthcare providers with a new prosthetic option, one that may provide benefits that outweigh the risks associated with the device and provide the patient with improved mobility and enhanced quality of life.
Further Reading:


