Now that medical device manufacturers have begun to implement MDR-compliant post-market surveillance (PMS) systems, it is a good time to take stock of common areas of difficulty and inefficiency, and consider how best to address these. Even manufacturers who are continuing to place their devices under extended MDD certification have been required to comply with MDR post-market requirements since May this year.
One common issue we’re seeing is that manufacturers are unsure where to fit PMS activities alongside data flows from other EU MDR activities within their Quality Management System. Since the EU MDR is written from a risk-based perspective, we have found it useful to present each activity around risk management, as depicted in the diagram below.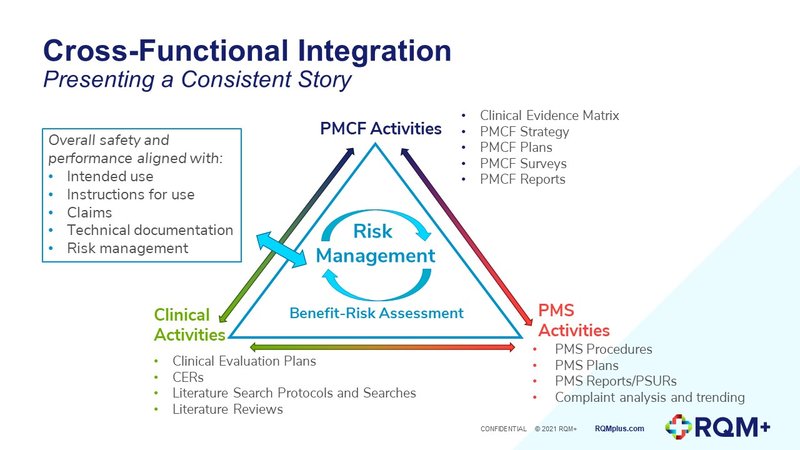 Where should manufacturers begin? In theory it is straightforward: you begin with the intended performances, clinical benefits, product claims and outputs of your risk management (RM) process as part of the clinical evaluation plan (CEP). This sets the context of the clinical evaluation and the framework for what you are trying to demonstrate. The post-market surveillance (PMS) and post-market clinical follow up (PMCF) plans are then defined by the residual risks identified by the clinical evaluation report (CER) conclusions. The execution of the PMS and PMCF plans provides data which then flow back into the process, following a classic plan-do-check-act cycle, triggering updates to the RM, CEP, CER and PMS and PMCF plans as required.
Where should manufacturers begin? In theory it is straightforward: you begin with the intended performances, clinical benefits, product claims and outputs of your risk management (RM) process as part of the clinical evaluation plan (CEP). This sets the context of the clinical evaluation and the framework for what you are trying to demonstrate. The post-market surveillance (PMS) and post-market clinical follow up (PMCF) plans are then defined by the residual risks identified by the clinical evaluation report (CER) conclusions. The execution of the PMS and PMCF plans provides data which then flow back into the process, following a classic plan-do-check-act cycle, triggering updates to the RM, CEP, CER and PMS and PMCF plans as required.
In practice, however, the cycle may not always be this straightforward, since new clinical objectives or hazards may appear during each stage of the process. This is to be expected; the important thing is having a procedure and document control system that shows how you are managing all of these inputs and changes.
Additionally, there may be multiple people and functions involved in each area of data collection and analysis. It is critical to make sure each stakeholder is aligned on the documentation that needs to be generated and the appropriate format. This planning of the flow of information and aligning the inputs and outputs between functions should take place as early as possible to reduce inefficiencies and ensure consistency in the final reports.
My colleagues and I will be speaking in a panel discussion about PMS implementation challenges and solutions at the MedTech Summit, including a look at common Notified Body findings so far. We'll also be hosting a roundtable discussion on IVDR Implementation, so be sure to stop by both sessions with your questions for us.
Where to find us at MedTech Summit
Stop by our virtual showcase hub for access to premium content and be sure to attend our expert panel discussions:
PMS Requirements of the EU MDR: Implementation Challenges and Solutions
Wednesday, 20 October 2021 at 15:10 - 15:50 CET/CEST
Join our session and have your questions answered live by a panel of former Notified Body (NB) leadership and PMS experts. Panelists in this session will also discuss:
- Best practices for optimizing every element of PMS/PMCF as it relates to the EU MDR at your organization
- What typical NB findings have been so far and how interpretations may vary related to PMS requirements
- Strategies to overcome audit findings and gaps in your evidence
Panelists:
- Amie Smirthwaite BEng, PhD, RQM+ Senior Vice President, Intelligence & Innovation
- Celeste Maksim, PhD, RAC, RQM+ Chief of Staff, Clinical & Post-Market Practice
- Ed Ball CEng MIPEM MIMMM, RQM+ Senior Associate
- Andrew Tarnaris, M.D. MD(Res) FRCS(NeuroSurg), RQM+ Medical Director
- Moderated by Lisa Casavant, RQM+ Executive Vice President
IVDR Implementation Best Practices and Challenges Roundtable
Thursday, 21 October at 17:20 CET
Learn from the experiences of seasoned IVDR experts – including Notified Body perspectives – as they share their IVDR implementation successes and failures so far, along with answers to your questions. Our expert panel has worked through comprehensive strategies and solutions and is prepared to share their learnings and tips for success.
Panelists:
- Amie Smirthwaite BEng, PhD, RQM+ Senior Vice President, Intelligence & Innovation
- Celeste Maksim, PhD, RAC, RQM+ Chief of Staff, Clinical & Post-Market Practice
- Nancy Morrison, RAC, RQM+ Executive Director, Regulatory & Quality Services
.png?width=600&height=314&name=Social%20Media%20Banner%20-%20Integrating%20Post-Market%20Surveillance%20(1).png)


