Retrospective PMCF Studies
When your post-market clinical follow-up (PMCF) plan includes retrospective PMCF studies, specialized expertise is required to develop and implement these studies to generate meaningful data. RQM+ has the clinical and regulatory expertise to create and execute retrospective PMCF studies so your teams can focus on what they do best.
“User feedback studies were like my arch-enemy before RQM+."
- Sr. Clinical Program Manager, Multinational Medical Device Manufacturer
We gather inputs to determine the study goals and tailor questions to clinical outcome measures. From this, we develop study questions, align them with regulatory and ethical considerations, apply study design best practices, and cross-reference with regulations and guidance.
We handle the practical logistics of deploying retrospective PMCF studies and gathering data so your team can focus on daily operations. This includes the electronic deployment of studies to qualified users, response reporting, and managing honoraria when applicable.
Our team will analyze study responses and pass on negative feedback that could influence product design, risk management, and quality systems. We provide analysis sets for every study deployed so you have a complete data set.
We generate retroactive PMCF study reports that describe the rationale behind the study design and include results and analysis. The conclusion of the retrospective PMCF study is then copied into CER, PSUR, and PMCF reports so all of your documentation is in alignment and compliant with regulatory requirements.
Comprehensive Retroactive PMCF Study Development and Execution That Aligns with Your Business Goals
We do not just write studies—we add value to your business with every project.
-

An Integrated Approach to Study Development
Our expert team is laser-focused on telling a consistent story about your product. We do this by aligning study objectives and clinical outcomes from the clinical evidence report, adverse event questions and language from the instructions for use, and questions on new or emerging risks from PMS data. We create response scales that align with clinical practice and enable comparison of results to the risk management file and benefit-risk assessment.
-
Retrospective PMCF Study Best Practices
When developing retrospective PMCF studies, we employ best practices and work closely with your team to gather inputs and perform a clinical evidence analysis from CERs, PMS data, and your risk management file. We provide guided ideation sessions to develop study questions, tailor them to clinical outcome measures, and create a custom response scale flowchart. The review process takes place in an online platform so every team member can provide input and work collaboratively.
-
A Business-Balanced, Collaborative Approach
As a strategic partner that always has your business goals in mind, we look for ways to add efficiencies across your portfolio and seek to identify areas of clinical data overlap in existing data, align end points of data to be collected, and bundle products in a single study or PMCF activity when possible.
-
Validated Retrospective PMCF Study Execution
At RQM+, we have already seen feedback on retrospective PMCF studies from notified bodies and are familiar with the expectations of notified bodies. With every project, we make process improvements based on lessons learned so you can be confident that we are applying the most current best practices to your studies.
Retrospective PMCF Study Development and Execution FAQs
Hear a discussion about data quality and ranking the quality of data coming from a study in our RQM+ Live! #44 show.
Under what circumstances are retrospective PMCF studies the solution?
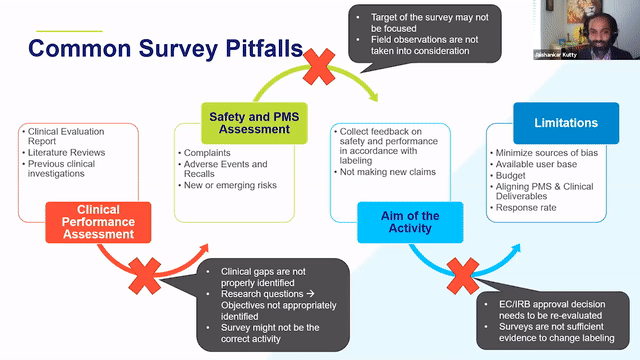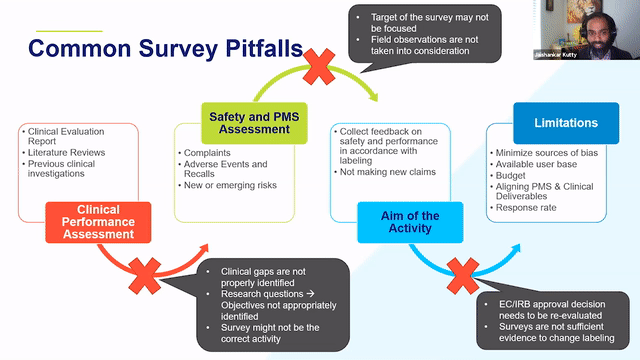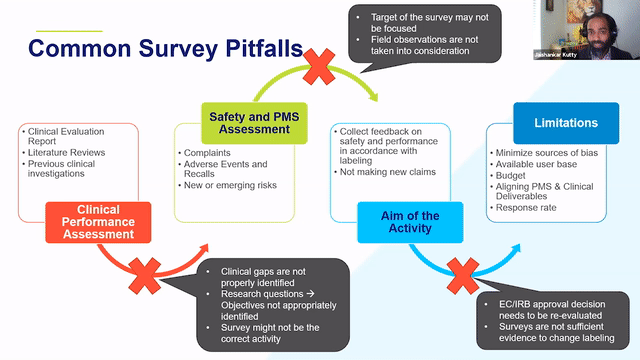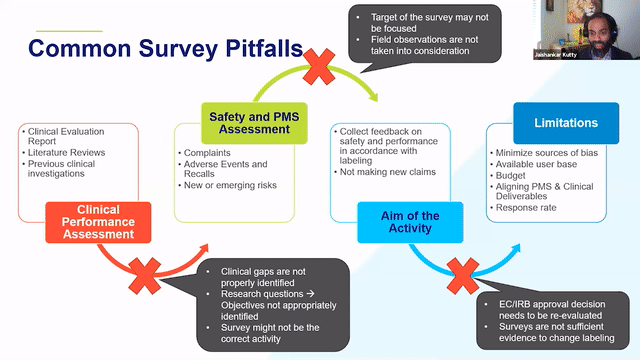
When thinking about retrospective PMCF studies, it is a good idea to think about the clinical evidence, CER, history of the device from a post-market side as well to identify the areas of gaps and the areas where the device can be improved and then think through whether or not a survey would address those needs.
GLOBAL BOTTOM CTA INSTRUCTIONS:
To display custom copy instead of global copy in this section, please go to Show Global Content for Bottom CTA? toggle in the "Contents" tab to the left, toggle it off, save, and then REFRESH the page editor, the custom text will then show up and ready to be edited.
Turning the global content back on will be the same process, go to the toggle and toggle it back on, save and refresh!