Quality System Integration: CERs/PERs, Risk Management, and Post-Market Surveillance
EU MDR and IVDR have transformed the industry, and many manufacturers are struggling to update their systems. The clear integration of PMS, CERs/PERs, and risk management is critical for meeting notified body expectations and keeping products on the market. RQM+ has extensive expertise in successfully integrating these systems to optimally support business operations.
We help global manufacturers create and implement procedures and strategies for writing clinical/performance evaluation and post-market surveillance plans and reports for medical devices and diagnostics (CEPs, CERs, SSCPs, PEPs, PERs, SSPs, PMS Plans and Reports, PSURs, PMCF, PMPF ). For many manufacturers, having audit and submission ready CERs and PERs is on the critical path to certification, and these documents are expected to meet intense scrutiny by the notified bodies. We will develop and implement procedures and processes that optimize efficiency for your team and have you ready for MDR/IVDR certification.
Technical documentation is an overarching requirement that touches all aspects of regulatory and quality compliance. In addition to the initial effort to create your submission package, documents must be continually updated as new information becomes available. We will make sure all of your systems are integrated so technical documentation can be efficiently maintained.
RQM+ provides comprehensive post-market surveillance consulting services that include strategy and implementation of plans and reports for PMS and post-market clinical follow-up (PMCF) or post-market performance follow-up (PMPF). All of these documents rely on data from clinical efforts, which is why integration is so important.
Risk management is an underlying system that must be updated based on new clinical and PMS information, including ongoing benefit-risk analyses as the data evolves. Having all of these systems in alignment through cross-functional integration makes it possible to share the full story with notified body reviewers.
RQM+ Helps You Tie It All Together
When departments and systems do not align, our team will develop and implement an integration plan.
-

Cross-Functional Integration Services
The goal of cross-functional integration is to ensure that overall safety and performance are aligned with the intended use, instructions for use, claims, technical documentation, and risk management. With multiple departments that have their own priorities and systems, we know how challenging it can be to get your internal systems to align.
-
RQM+ Project Management
Quality system updates to meet MDR and IVDR requirements—especially the integration of risk, PMS, and clinical elements—can feel overwhelming and impossible to get right the first time. Our dedicated project managers who focus only on QA/RA projects will implement an efficient project timeline and smooth transition, putting your mind at ease by ensuring all impacted departments are working in alignment.
-
Integrated Review of Systems
The seasoned professionals at RQM+ know how to ensure the necessary linkages between technical documentation, clinical evaluation documents, PMS documents, and risk management documents in order to improve alignment and increase efficiency.
We will review your existing systems to identify areas for improvement and develop optimized procedures that set the foundation for compliance. Optimization ensures that the same data is not being generated or reported in different ways across functions, which leads to wasting precious resource time and potential confusion in audits. Our collective knowledge and best practices will be incorporated into a system that is customized for your business.
-
Validated Tools and Processes
With thousands of projects under our collective belts, we have truly seen it all. This has allowed us to develop and optimize tools and best practices that help you efficiently and consistently achieve compliance. We know our approach is effective because the results have been validated by notified body client audits.
RQM+ is at the forefront of regulatory and quality compliance.
With decades of experience and deep industry connections with various notified bodies and health organizations worldwide, RQM+ is a leading source for industry insights and best practices.
Quality System integration FAQs
The integration of clinical, regulatory, risk management and post market surveillance is a difficult and confusing task. How should the data flow and where does it start?
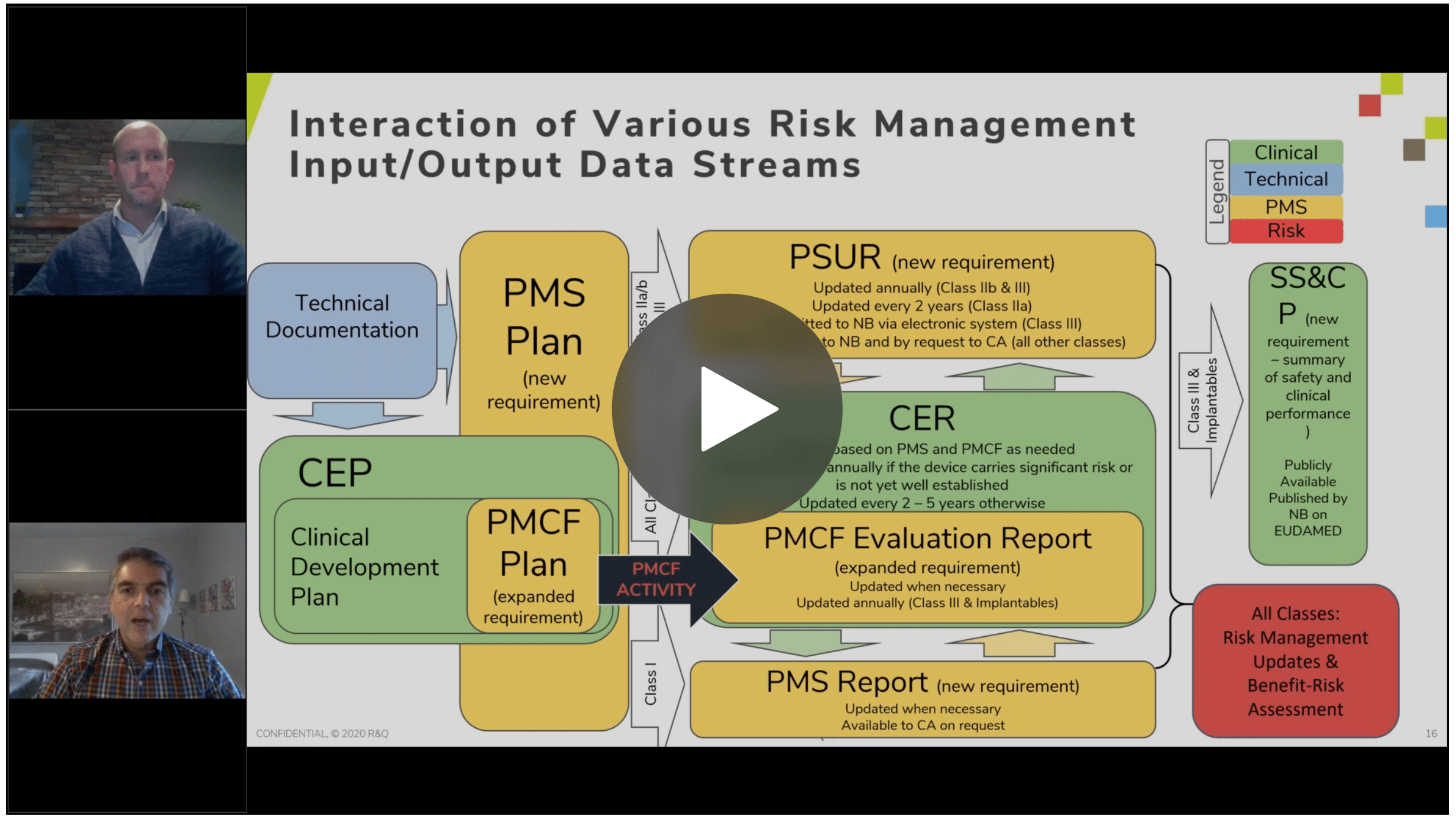
The video FAQ provides a helpful graphic along with our subject matter experts describing the cross-functional integration of the QMS and how the data flows between PMS, PMCF, and clinical activities, Where do we start? It looks straight forward - you have a new product with claims and a risk analysis, flowing into a CER, which flows into a PMCF plan and then data from PMS and PMCF activities flow back to the risk analysis which gets updated. However, rather than being a smooth wheel like what was just described, it's more like a wheel with eddies in it, or cycles within a cycle, and that's expected. The important thing is having a procedure and document control system that shows how you're managing all of these inputs and changes.
Do all residual risks identified need to be listed in your IFU and SSCP?
It's not feasible to identify and list all risks, but knowing where to draw the line can be difficult. Per the GSPRs, every residual risk should be listed. The notified bodies are using a pragmatic approach in assessing whether all of the important risks have been listed, but the interpretation can vary by notified body reviewer. In this video FAQ, our former notified body reps discuss factors they considered and a couple examples.
Who decides what residual risks should be listed and could the NB push for a specific risk?
At the end of the process, the NB issues a certificate, but it's the manufacturer who signs the DOC and it is the manufacturer's responsibility to define what is appropriate. The manufacturer knows the device and intended population the best, but the information must be clearly documented in the risk management process for review by the NB. The NB may raise a gap identified in the review process and push to add a residual risk, and that will need to be resolved with the manufacturer during the Q&A process.
One factor in ensuring your risk assessment is comprehensive is to utilize a cross functional team to appropriately identify and agree on residual risks. It cannot be done effectively with just a risk manager or just a clinical person - it takes a team.
Tap into the RQM+ Knowledge Center

FAQ
Why should I build a quality system backwards?
Webinar
Integrating Risk and Complaint Management

Technical Brief
ISO 14971: 2019: 3 Key Changes from ISO 14971: 2007
FAQ
The integration of clinical, regulatory, risk management and post market surveillance is a difficult and confusing task. How should the data flow and where does it start?
GLOBAL BOTTOM CTA INSTRUCTIONS:
To display custom copy instead of global copy in this section, please go to Show Global Content for Bottom CTA? toggle in the "Contents" tab to the left, toggle it off, save, and then REFRESH the page editor, the custom text will then show up and ready to be edited.
Turning the global content back on will be the same process, go to the toggle and toggle it back on, save and refresh!


