Delivery Models
Your vision, our versatility.
RQM+ offers two delivery models to align with your project's needs and ensure optimal outcomes.
We currently offer this for clinical evaluation, performance evaluation, post-market surveillance, and post-marketing clinical follow-up deliverables associated with EU MDR and IVDR.
Every project is unique.
That's why RQM+ has diversified our EU MDR and IVDR support with two distinct service packages—each designed to match your project's specific demands and budget.
Designed for You. Always.
-

Full Service
Our Full Service model is the gold standard for clients seeking a robust support system for EU MDR and IVDR clinical regulatory affairs challenges and is the best model for most clients. It offers comprehensive project management, strategic guidance, and an individual or team of technical experts.
Ideal for clients who require:
– In-depth project management
– Strategic insights
– Collaborative team approach
– Support for complex regulatory situations -

Value+
With Value+, experience a streamlined yet powerful approach to creating EU MDR and IVDR clinical affairs documents. Offering senior-level expertise at 30% less cost than our Full Service model, Value+ is ideal for clients who need:
– High-quality, cost-efficient documents
– Senior-level expertise without additional subject matter expert and project management support
– Solutions for straightforward regulatory needsClients who meet the following criteria are typically a great fit for Value+:
– Have a limited budget
– Just want a document and a writer
– Have final input documents available
– Able to commit to an efficient timelineWe offer this service for document updates, as well as new documents.
MDR implementation has rocked the industry, leaving many manufacturers heading into notified body reviews with much uncertainty.
We provide all the resources—people, processes, and technology—to create exceptional clinical evaluation documentation. Even the notified bodies agree. One client said, “They [the notified body] loved the reports, loved the evidence matrix, and said they were the best CERs they have seen by anyone to date.”
Clinical Roadmap
Our experts will help you develop a clinical evaluation strategy to achieve your business objectives and notified body approval.
We provide innovative strategies for using existing data along with your PMCF plan based upon our experience with a wide range of notified bodies.
From CEPs to SSCPs, we enable you to tell a compelling story, making your documentation appealing to regulators.
Our integrated approach reduces time to certification with fewer notified body questions and nonconformities.
Quality System Solutions
CERs cannot be created in a bubble by the clinical team. There are inputs and outputs between clinical, post-market surveillance, and risk management. Integrating the processes optimizes resources and reduces the risk of audit findings. Our experts will help you update and revise your quality system so that all your processes are working together efficiently. This will ensure that you can meet the requirements of the MDR now and in the future.
NB Response Support
The notified body may not interpret the MDR's requirements in the same way you do and you only have three rounds of Q&A to gain alignment and approval. Additionally, a deficiency that calls for more clinical data could result in an expensive study or the loss of product certification. With so much on the line, a different perspective and proposed response plan from our former notified body leaders and subject matter experts could make all the difference.
CER Remediation
The RQM+ team will assess your CER process and documentation by product line, providing you with a detailed gap analysis and remediation plan. Our team of experts can effectively execute a remediation of any size, minimizing the burden on your team so you can maintain focus on new products.
Our CER project managers act as the single point of contact for all client needs and are responsible for leading meetings, developing schedules for deliverables, monitoring progress, and driving results. This includes actively managing the client approval process and coordinating independent review of all documentation.
For ongoing compliance, we ensure client documentation is updated as new data becomes available and then provided to notified bodies by the required deadlines.
Clinical evaluation preparation requires a deep understanding of medical devices, clinical data, and what is sufficient to prove safety, performance, and state of the art. RQM+ can take the lead so you do not have to.
01 Expert Strategy and Execution
Whether you need targeted support for specific tasks or comprehensive technical leadership and project management, RQM+ can help with every aspect of the clinical evaluation process.
02 Ongoing Support
Clinical evaluation is a continuous effort requiring integrated solutions. RQM+ is here as long as you need us.
03 Proven Success
We consistently meet reviewer expectations because we know what they want to see. Positive feedback from notified bodies proves it.
04 Former Notified Body Leadership
RQM+ leadership and staff includes people who have drafted MDCG guidance documents on clinical evaluation, device equivalence, and sufficient clinical evidence for legacy devices.
Featured Leader

Amie Smirthwaite, Ph.D.
Senior VP, Intelligence & Innovation
Dr. Amie Smirthwaite joined RQM+ in March of 2020. A clinical and regulatory affairs expert, Amie has over 25 years of postdoctoral experience in medical devices, spanning new product development, quality and regulatory systems, and clinical data evaluation. She is leading the RQM+ Intelligence and Innovation team and brings a wealth of knowledge and experience having worked for medical device companies, academic institutions, and BSI.Entrusting your CERs to a consulting firm requires confidence in the team. With RQM+, you get a level of clinical and regulatory expertise and client dedication you won’t find anywhere else.

More FAQs About MDR Clinical Evaluations
What should we do for the clinical dev plan req for a legacy device? For example, if we don't plan to do a new clinical study for a legacy device that has no new risks that need to be addressed, what should be in the plan?
In MDCG 2020-6 guidance, it acknowledges that for a legacy device that's been on the market for a while, it's not appropriate to recreate the development plan because you've already been through that process. Instead, per the appendix to the guidance shows what you should have in lieu of having new clinical data, including a PMS plan.
Can you have a CER with multiple products, some of which are under the MDR and some that will remain under the MDD until a later date?
From the NB point of view, you could write one CER that covers both the MDR and MDD requirements, and be clear so that the NB can see the delineation. This can be complicated to maintain, and require a lot of mapping and explaining, so it may be easier to separate the products.
Tap into the RQM+ Knowledge Center
Event Recording
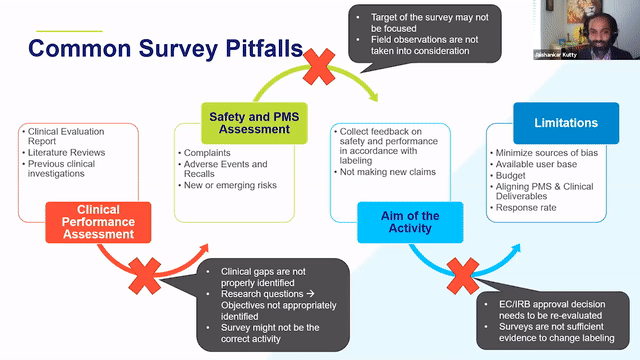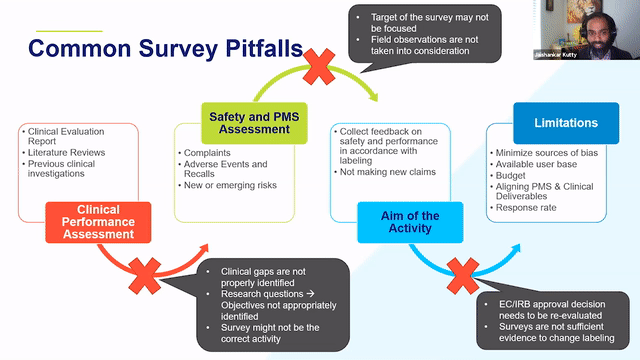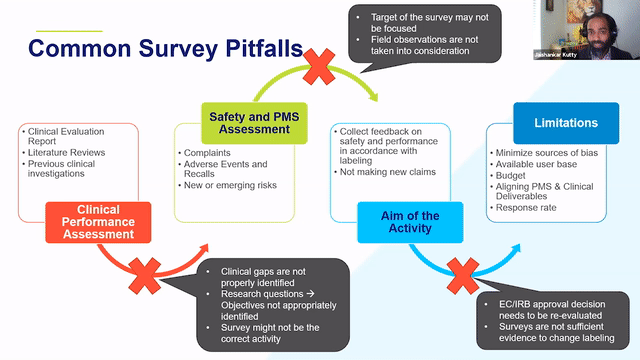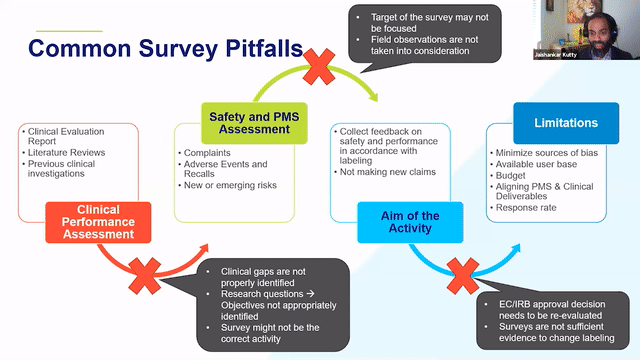
RAPS + RQM+ Webcast —PMCF User Feedback Surveys

Technical Brief
How to Create a Compliant Periodic Safety Update Report (PSUR) Under EU MDR and EU IVDR

Whitepaper
PMCF Under MDR
FAQ
The integration of clinical, regulatory, risk management and post market surveillance is a difficult and confusing task. How should the data flow and where does it start?

Webinar
Best Practices in Scientific Database Searching

Webinar
Best Practices for Integrating CERs and PMS Under MDR
GLOBAL BOTTOM CTA INSTRUCTIONS:
To display custom copy instead of global copy in this section, please go to Show Global Content for Bottom CTA? toggle in the "Contents" tab to the left, toggle it off, save, and then REFRESH the page editor, the custom text will then show up and ready to be edited.
Turning the global content back on will be the same process, go to the toggle and toggle it back on, save and refresh!









