3 September 2020 - Training Workshops brought to you by the Subject Matter Experts at Regulatory & Quality Solutions.
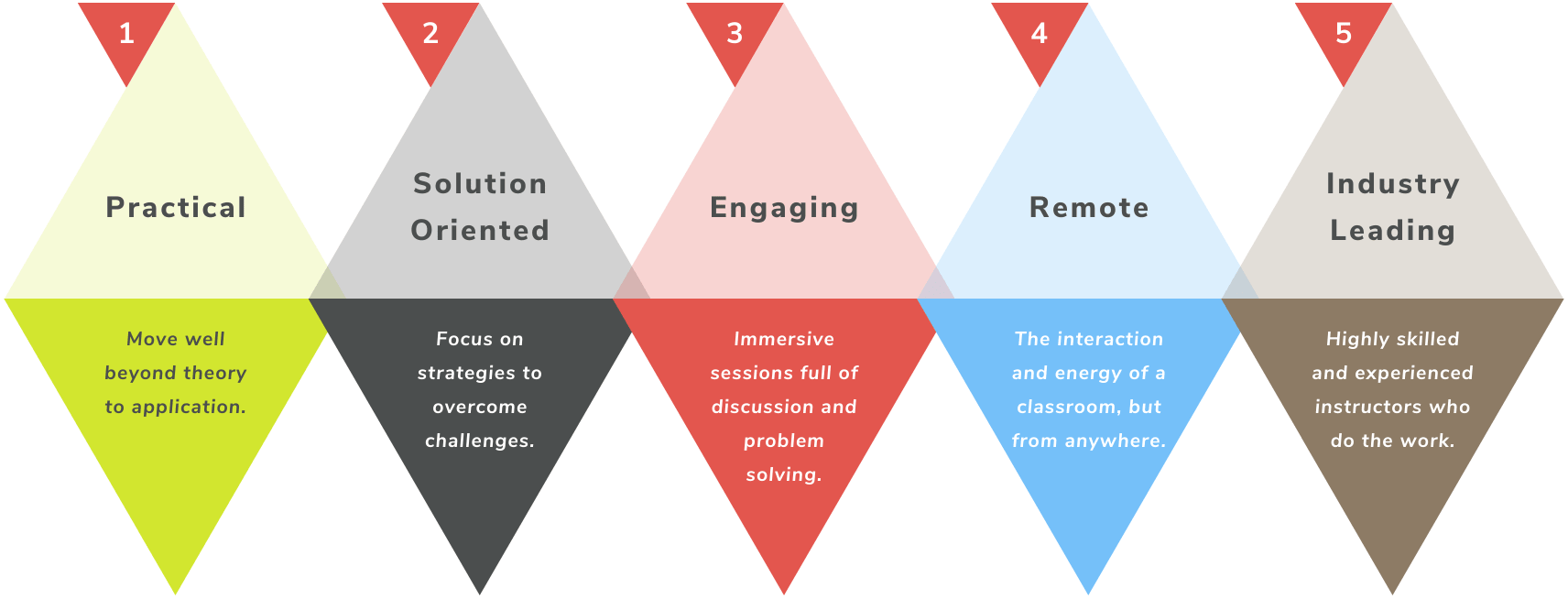
R&Q now offers industry-best, comprehensive live training workshops to help your team and organization develop applicable skills and outperform expectations. Taught by R&Q Subject Matter Expert virtual instructors, our industry-leading faculty provides in-depth training on the knowledge and skills you need to tackle the most pressing medical device manufacturing global regulatory and quality challenges.
The Benefits of R&Q's Training Workshops
Current Offerings
For pricing, please submit the form on the workshop page you are interested in and we will contact you.
EU MDR for QMS Auditors:
|
Auditing Skills:
|
|
MDSAP:
|
|
EU MDR:
|
Don't see what you need?
R&Q will customize a live training workshop just for you.
Let us know what you're looking for by contacting us today.
R&Q Faculty Spotlight
 Paul Briest Operations Manager, R&Q |
Paul is an Engineering and Six Sigma professional with over 20 years of experience in managerial, quality, manufacturing, development, and project management roles including eight years with Beckman Coulter, a world leader in the in vitro diagnostics market. Paul is a subject matter expert in the areas of design controls, risk management, quality systems, product quality, and post-market surveillance for in vitro diagnostic devices Find Paul in these R&Q Training Workshops: |
 Niki Caporali-Spaniel, RAC Principal Specialist, R&Q |
Niki has been a key contributor to the success of R&Q and its clients since 2010. With over twenty years of medical device industry experience, including several years in lead roles in quality management system creation and maintenance, she has acquired extensive skills and experience in project management, clinical trials, quality management system development and implementation, complaint handling and quality auditing and training. Niki’s expertise also includes regulatory affairs in which she has provided both strategic and hands-on support for various regulatory submissions including IDE’s, Pre-Submissions, and 510(k) for Class I, II, and III devices. Niki is also skilled in technical writing and has used this along with her regulatory knowledge to develop clinical evaluations reports in support of products sold in the EU. Find Niki in this R&Q Training Workshop: |
 Felicia Hosey, RAC Senior Principal Specialist, R&Q |
Felicia is has worked in the medical device industry for over 15 years acting as a researcher on developmental projects, key product development and regulatory project team member and regulatory consultant to a wide variety of medical device and in vitro diagnostic medical device companies. Felicia is a member of RAPS with a global RAC, which has equipped her with the skills necessary to be successful on projects relating to domestic and international medical device regulations. She is also a member of the R&Q Leadership Council which is responsible for preparing reference tools and templates for use with multiple devices types during EU MDR and IVDR remediation. Find Felicia in this R&Q Training Workshop: |
 Celeste Maksim, Ph.D. Principal Consultant, R&Q |
Dr. Maksim is a Principal Consultant with Regulatory & Quality Solutions (R&Q) where she helps to provide clients with industry-leading regulatory and quality consulting and engineering services throughout the entire product lifecycle. Her medical device experience spans from research and development of nanoscale electrochemical sensors for emergency medicine applications and nanomaterials that enable drug delivery across the blood brain barrier to regulatory experience in generating regulatory strategies, the development of FDA submissions, including IND, NDA, IDE, and 510(k) submissions, biocompatibility strategy, and EU MDR preparation focusing on post-market surveillance and post-market clinical follow-up. Celeste is Regulatory Affairs Certified (RAC) by RAPS, possesses a Bachelor’s degree in chemistry from Northern Kentucky University, and a PhD in Analytical and Materials Chemistry from Indiana University, Bloomington. Find Dr. Maksim in this R&Q Training Workshop: |
 Rem Siekmann Senior Principal Engineer, R&Q |
Rem is a QMS auditing expert who has performed over 300 ISO 9001/ISO 13485 audits. He's also a Medical Device Technical Manager for a certification registrar and Certified Biomedical Auditor. Rem has extensive experience with MDSAP, having achieved MDSAP Auditing Organization status, formally trained to the quality and regulatory process, and has created and trained numerous organizations on the R&Q ISO 13485/MDSAP audit tool. Rem is also a product development expert, having successfully brought over 15 new medical devices from concept to full-scale introduction both in R&D and product management roles, including two US patents. Find Rem in these R&Q Training Workshops: |
 Ibim Tariah, Ph.D. Vice President of EU MDR and IVDR Consulting Services, R&Q |
Dr. Tariah earned his PhD in Materials Science the University of Manchester, U.K. and has over 30 years experience in the medical device industry, including 21 years at BSI. In Dr. Tariah's role of VP of EU MDR/IVDR Consulting Services at R&Q, he provides leadership, strategic advisement and implementation expertise to R&Q's consulting team and medical device clients. Prior to R&Q in his role of Technical Director of Medical Devices for BSI Americas, Dr. Tariah was part of the BSI Americas leadership team responsible for transitioning from MDD to MDR certification. Dr. Tariah recognized the significant challenges in implementation of the EU MDR. He created and led customized MDD and MDR workshops for clients and industry, and spoke on behalf of the notified body at global conferences and events. Find Dr. Tariah in this R&Q Training Workshop: |
 Carol Vierling, RAC Senior Principal Advisor, R&Q |
Carol has over 30 years of regulatory and clinical experience in the medical industry with US and global regulatory submission experience. The previous three years have been with R&Q working with start-up, mid-size and multi-national medical device companies. Carol has a Bachelor of Science in Nursing, a Masters in Business Administration and RAC certification. Carol has been a member of RAPS for more than 20 years. During that time, she has authored an article for RAPS Focus and was a member of the Board of Editors for five years where she has been a reviewer and topic leader. Find Carol in this R&Q Training Workshop: |