Looking back to 2020, as the COVID-19 pandemic gripped the world, much of the FDA’s focus turned to their response to the public health emergency. CDRH in particular reviewed thousands of emergency use authorization (EUA) applications and worked to facilitate the development and availability of COVID-19 tests and collection kits, personal protective equipment (PPE), ventilators, and other devices. Despite this shift in focus, CDRH also received an increase in “conventional” premarket submissions, such as 510(k)s, De Novos, Premarket Approvals (PMAs), Q-Submissions (Q-Subs), and surprisingly even IDEs. Overall, this amounted to a massive 38% increase to CDRH’s premarket submission workload).1
The abrupt and urgent impact of COVID-19 placed a strain on the Center, which resulted in submission review delays, refusal of Q-Subs, disruptions in clinical studies and post-market compliance activities, and reprioritization of non-pandemic related tasks. Despite these challenges, this pandemic showcased many of FDA’s strengths and a willingness for flexibility. FDA was able to disseminate critical information through the release of numerous COVID-19-related guidance documents and town halls, increase their safety surveillance activities, and implement more flexible review practices to allow life-saving devices to reach patients in an expedited process.
In 2021, we started to see CDRH start to emerge from all the EUAs and pandemic related work. Whereas last year when it was all hands-on deck and reviewers from all OHTs were getting asked to review COVID-19 related work, it is now seeming that the COVID-19 products are localized to a few Offices of Health Technology (OHTs). For the other OHTs, it seems like things are getting back to business. New programs are being launched, Guidance Documents released, Q-subs are being accepted, and there is even talk about going back to the office in early 2022 (although the Omicron variant may impact this goal). As 2021 has come to a close, we would like to provide an analysis of the 2021 MDUFA numbers to quantify how FDA is really doing as well as provide a brief recap of some of our major 2021 takeaways in this technical brief! Also, join us on January 13 for RQM+ Live! #46: Year-end Review and 2022 Outlook on FDA Submissions.
Please note that all figures and references in this post have been obtained directly from MDUFA IV (FY2018-2022) Performance Report. See the full report here.
1. Lingering effects of COVID-192
While 2021 saw the start of a return to normalcy for CDRH, the impact of COVID-19 continued to linger and has not affected all of CDRH evenly. Some OHTs have returned to business as usual, while others continue to experience a significant increase in workload and delays. To quantify the impact of the pandemic, we reviewed the 4th Quarter FY 2021 MDUFA IV Performance report, released on November 16.3 Although the 2021 numbers are still incomplete, enough data have been reported to provide insight to the status of FDA.
The major outliers we see so far lie in the 510(k) and Q-Sub performance data. PMA, De Novo, and IDE numbers for 2021 (e.g., number of submissions received, review timeline performance) appear roughly equivalent to previous years across the Center (which highlight both Industry and FDA’s focus to these innovative products. See Theme 2 below!). Throughout the pandemic, FDA has had to prioritize their COVID-19 related efforts ahead of innovative products at large (e.g., Breakthrough, STeP, PMA, De Novo, and IDE submissions), followed by MDUFA tracked items (510(k)s), then lastly non-MDUFA items (e.g., Q-Subs, guidance documents unrelated to COVID-19). This is especially evident from the 4th Quarter FY 2021 MDUFA IV performance numbers.
510(k) results
Both 2020 and 2021 saw a significant increase in 510(k) submissions missing the MDUFA performance goal as well as a sharp decrease in review times (See Figure 1 below).
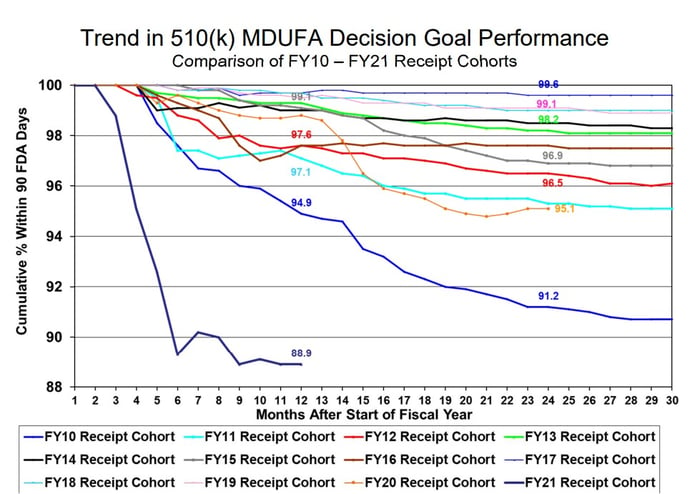 Page 119, MDUFA IV (FY2018-2022) Performance Report
Page 119, MDUFA IV (FY2018-2022) Performance Report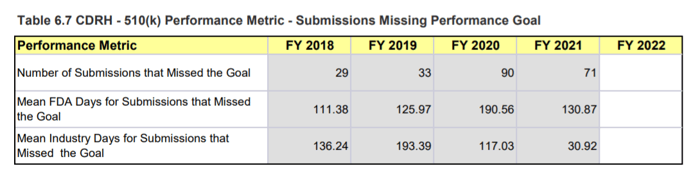 Page 134, MDUFA IV (FY2018-2022) Performance Report
Page 134, MDUFA IV (FY2018-2022) Performance Report
Figure 1: CDRH 510(k) Performance
While there appear to be fewer 510(k) submissions with missed goals in 2021 compared to 2020, it is important to note that there are still 1840 pending submissions at the end of the year with 189 of those submissions already exceeding 90 FDA review days (Figure 2). These appear to correspond to the highest number of year-end pending submissions since 2010, and the most pending with more than 90 FDA days in the last sixteen years. The large number of pending submissions indicates that CDRH will continue to see delays and missed performance goals heading into 2022.
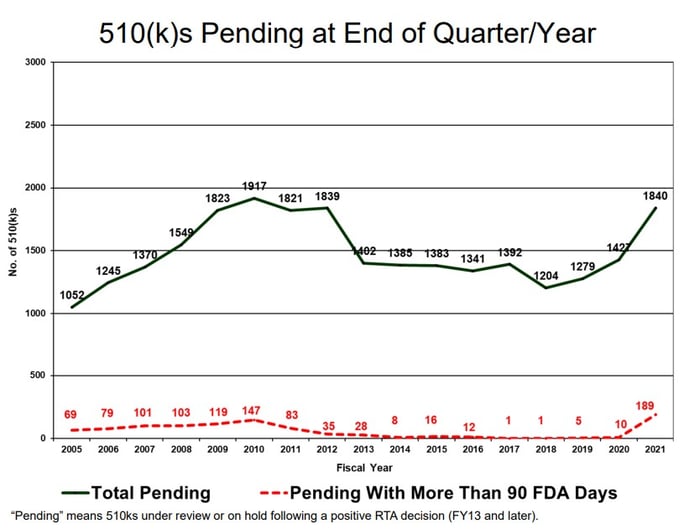
Page 120, MDUFA IV (FY2018-2022) Performance Report
Figure 2: 510(k)s still pending a final decision
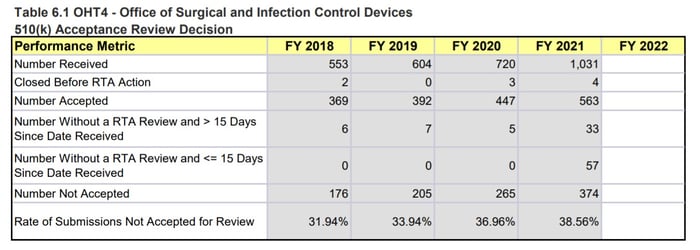
When looking at the specific OHTs, we notice the biggest differences in OHT 4: Office of Surgical and Infection Control Devices and OHT7: Office of In Vitro Diagnostics and Radiological Health. This comes as no surprise as both these groups have seen large influxes of COVID-19 related work - OHT4 is responsible for regulating devices like PPE, reprocessing, and disinfection devices, while OHT7 is responsible for in vitro diagnostics such as COVID-19 tests.
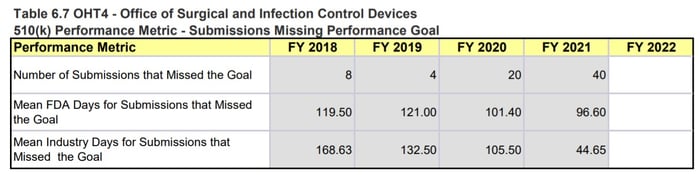
The OHT4 MDUFA performance metrics indicate that OHT4 has received a significant increase in the number of 510(k)s received in 2021. This almost 50% increase, which does not account for the increased workload due to COVID-19 response activities and other marketing submissions, has led to a steep drop in the performance percent metric (95.62% issued a MDUFA IV decision within 90 days in 2020 compared to 84.53% in 2021) and an increase in missed submission goals (4 in 2019, 20 in 2020, 40 in 2021). The tables in Figure 3 below also note that OHT4 has 57 submissions still in the queue awaiting an RTA decision with ≤ 15 days since the date received. That comprises ~5.5% of all the submissions they received in 2021 (57 out of 1031).
Page 151, MDUFA IV (FY2018-2022) Performance Report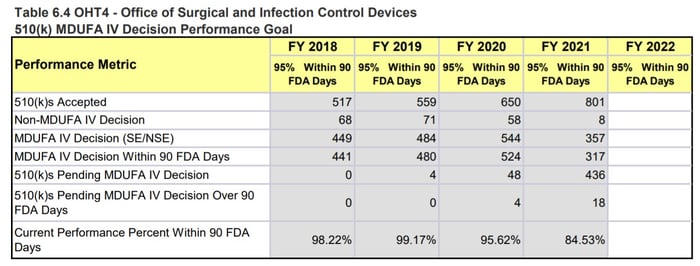
Page 152, MDUFA IV (FY2018-2022) Performance Report
 Page 154, MDUFA IV (FY2018-2022) Performance Report
Page 154, MDUFA IV (FY2018-2022) Performance Report
Figure 3: OHT4 510(k) Performance Metrics
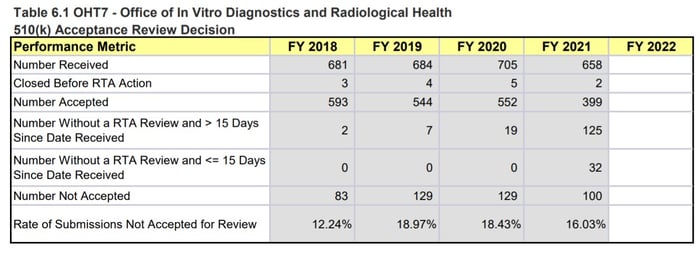
OHT7 is facing a similar situation and while the 510(k) numbers don’t seem as drastic as for OHT4, OHT7 is still seeing an abnormally high number of submissions with missed goals and increased review timelines. It is also important to note that these 510(k) numbers are just one metric for how the OHT is doing. For example, OHT7 was the only Office to explicitly state that they were only accepting certain pre-subs, meaning they must be dealing with more problems than what is reflected in the MDUFA numbers.
 Page 166 , MDUFA IV (FY2018-2022) Performance Report
Page 166 , MDUFA IV (FY2018-2022) Performance Report
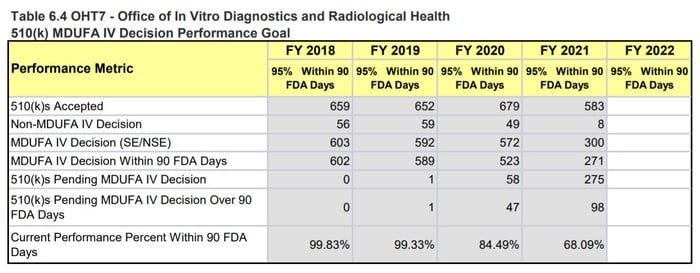
Page 167, MDUFA IV (FY2018-2022) Performance Report
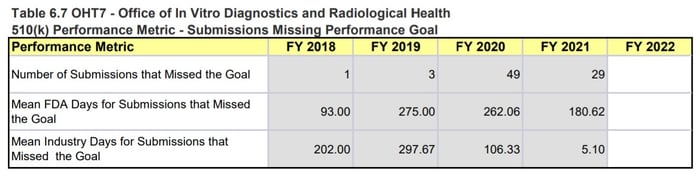
Page 169, MDUFA IV (FY2018-2022) Performance Report
Figure 4: OHT7 510(k) Performance Metrics
Pre-submission
In 2020, Q-Sub meetings were one of the first activities to be reprioritized. We saw Q-Subs closed without feedback and major delays in receiving feedback or scheduling meetings. In 2021, we see this effect continuing. According to the 4th Quarter MDUFA report, ~12.5% of all Pre-submissions were closed before RTA action (385/3086). These numbers include Q-Subs closed due to reallocation of resources to COVID-19 activities. Additionally, the length of time to schedule meetings has continued to creep up across the Center.
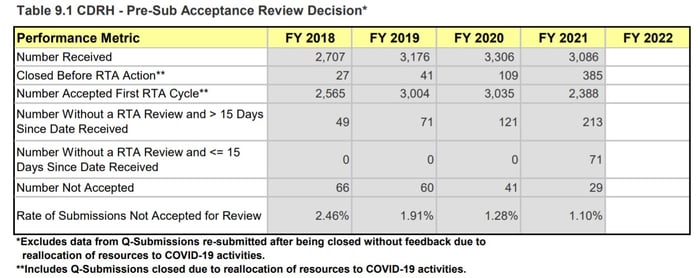 Page 215, MDUFA IV (FY2018-2022) Performance Report
Page 215, MDUFA IV (FY2018-2022) Performance Report
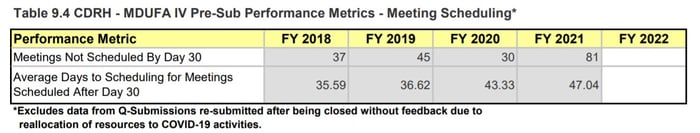
Page 215, MDUFA IV (FY2018-2022) Performance Report
Figure 5: CDRH Pre-sub Metrics
In looking at the specific OHTs, we again see a significant impact to OHT4 and OHT7, with both OHTs reporting a high number of Q-Sub closures. OHT1: Office of Ophthalmic, Anesthesia, Respiratory, ENT and Dental Devices also appears to be experiencing meeting scheduling delays, with the number of meetings not scheduled by Day 30 increasing from 10 to 17 and the average number of days to scheduling meetings increasing from 42 days to 62 days.
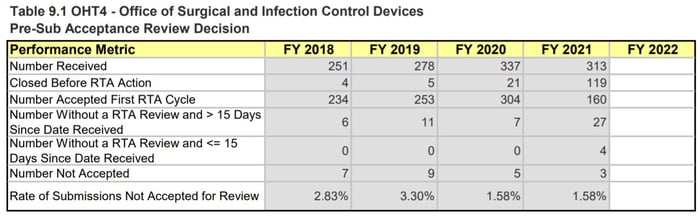 Page 220, MDUFA IV (FY2018-2022) Performance Report
Page 220, MDUFA IV (FY2018-2022) Performance Report
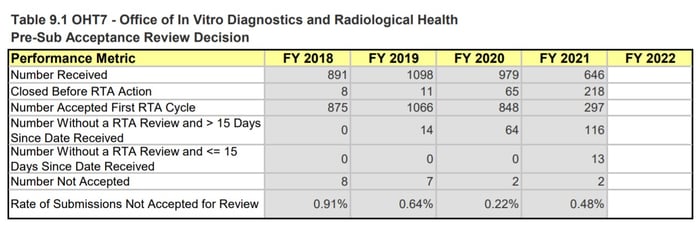 Page 223, MDUFA IV (FY2018-2022) Performance Report
Page 223, MDUFA IV (FY2018-2022) Performance Report
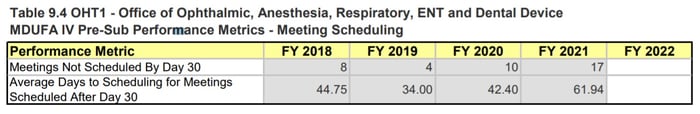 Page 217 , MDUFA IV (FY2018-2022) Performance Report
Page 217 , MDUFA IV (FY2018-2022) Performance Report
Figure 6: OHT1, OHT4, and OHT7 Pre-sub Metrics
Dr. Shuren has alluded to these delays throughout last year, stating in April 2021 that there would be review delays for those divisions reviewing PPE, ventilators, and general hospital equipment. Additionally, in 2021, he stated that OHT7 would only be only accepting pre-submissions related to COVID-19, companion diagnostics, breakthrough designation requests, or those which have a significant public health impact.
However, in a recent FDA Voices blog, released on December 21, 2022, Dr. Shuren announced that as of January 1, 2022, CDRH will also begin to accept IVD pre-submissions requesting feedback for submissions that would be likely to require a PMA or De Novo submission. He also notes that the Agency is hoping to be able to accept all IVD pre-submission requests by the late spring or early summer.4
These numbers only present one side of the story for how CDRH is doing. In our day-to-day, we feel how backlogged FDA is. We have received emails from reviewers late into the night, multiple delays in scheduling pre-sub meetings, and even had a pre-sub be rejected multiple times before being told to resubmit in 2022.
Even for the Q-subs that are being accepted, FDA has more frequently limited the number of questions or topics that can be discussed or provided more high-level feedback. While we hope CDRH is able to reduce the backlog, we anticipate similar issues going into 2022. It is incumbent on us as regulatory professionals to ensure our communications with FDA are succinct and are well formed in order to receive productive feedback in the current environment.
2. Focus on Innovative Devices
Despite the pandemic, the Breakthrough Devices program continued to receive priority attention from FDA. According to Dr. Shuren, FDA “approved, cleared, or authorized a record high of 132 novel medical devices in 2020, surpassing the 40-year high mark we set in 2018 and capping off 10 years of progress.”5
While some of these novel devices can be attributed to COVID-19 related EUAs, the number of innovative device submissions received by FDA continues to increase. There continues to be an upwards trend for Breakthrough Designation requests for innovative devices with nearly 250 Breakthrough requests submitted by June 2021.6
In addition to the growing Breakthrough Devices program, there have been several other updates affecting CDRH’s device innovation programs in 2021, with the most notable summarized below:
- In March, CDRH launched the Safer Technologies Program (STeP) for Medical Devices. This program is intended for medical devices that “significantly improve the safety of currently available treatments or diagnostics that target an underlying disease or condition associated with morbidities and mortalities less serious than those eligible for the Breakthrough Devices Program.”7 As a complement to the Breakthrough Devices program, STeP provides a new expedited pathway for innovative devices that focus on different diseases or patient populations and provides another mechanism to get safer treatments more quickly to the patients who need them.
- FDA issued a new final rule regarding De Novo classification requests and subsequently released four new Guidance documents related to De Novos. The new rule establishes procedures and criteria related to de Novo classification and provides additional requirements for De Novo classification requests.8
- The proposed Medicare Coverage of innovative Technology (MCIT) Final Rule for Breakthrough Devices has been repealed by the Centers for Medicare and Medicaid Services (CMS). The rule would have granted automatic Medicare coverage for four years to devices granted Breakthrough Designation by FDA and subsequently receive marketing approval or clearance. This rule would have made the Breakthrough designation even more enticing for manufacturers. Although this rule has been scrapped, thereby removing one of the incentives for innovative manufacturers to seek Breakthrough Designation, CMS has stated that they will continue to work with FDA to “develop an expeditious process to cover innovative devices that benefit Medicare patients.”9 FDA has been placing a priority on developing future partnerships with payors like CMS and other external stakeholder groups, as evidenced by meeting minutes from MDUFA V negotiation meetings, which will be discussed in a future RQM+ blog post.
- CDRH also continues to focus on the importance of digital health technologies as they expand their Digital Health Center of Excellence. In 2021, CDRH released multiple helpful documents for industry such as the “Artificial Intelligence and Machine Learning (AI/ML Software as a Medical Device Action Plan)” in September, and “Good Machine Learning Practice for medical Device Development: Guiding Principles” in October. With how quick the digital health space is evolving, there is an increasing need for guidance about how to design and regulate these products. To address this issue, CDRH has been training reviewers through the Experiential Learning Program (ELP) with the focus areas of Innovation and Digital Health, as shown in their training data reported in the MDUFA IV 4th Quarter Report (see Figure 7).
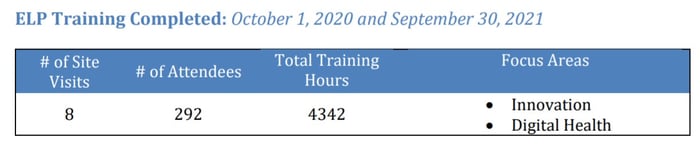 Page 347 , MDUFA IV (FY2018-2022) Performance Report
Page 347 , MDUFA IV (FY2018-2022) Performance Report
Figure 7: ELP Training Focus Areas for 2020-2021
3. Process Improvements
Another focus of FDA in 2021 was to improve their existing review processes. FDA launched the Electronic Submission Template And Resource (eSTAR) pilot and subsequently published a draft guidance about the template in September.10 The eSTAR pilot provides an electronic PDF submission template for 510(k)s and is structured to reflect the content and format of review templates used by CDRH reviewers. It is currently only a voluntary program with the aim to improve submission quality and thus decrease the total time to market.
The use of the eSTAR offers multiple benefits for companies: first, it allows an applicant to bypass the refuse-to-accept (RTA) review in favor of an initial technical screening, and the similarity to CDRH review templates may offer a more efficient review. In addition to the eSTAR template, FDA has introduced the 510(k) Progress Tracker, which allows companies to track the progress of their traditional 510(k)s.
Both these improvements come just in time before the final stages of MDUFA V negotiations – check back in on the RQM+ blog later this month for a preview of what to expect in 2022 and in the meantime join us on 13 January 2022 for RQM+ Live! #46 where our panel of experts will provide more insight on the Year-end Review and 2022 Outlook on FDA Submissions. Learn more and sign up here.
1 Shuren, J., & Maisel, W. (2021, April 15). A Year Into the Pandemic: How the FDA’s Center for Devices and Radiological Health is Prioritizing its Workload and Looking Ahead. Retrieved from U.S. Food & Drug Administration: https://www.fda.gov/news-events/fda-voices/year-pandemic-how-fdas-center-devices-and-radiological-health-prioritizing-its-workload-and-looking
2 Please note that all figures and references have been obtained from MDUFA IV (FY2018-2022) Performance Report:
3 MDUFA IV (FY 2018 - 2022) Performance Report - November 16, 2021 (4th Quarter). (2021, November 16). Retrieved from U.S. Food & Drug Administration: https://www.fda.gov/media/154217/download
4 Shuren, J., & Maisel, W (2021, December 21). Looking Ahead to 2022 as FDA’s Center for Devices and Radiological Health Manages a Sustained Increase in Workload. Retrieved from U.S. Food & Drug Administration: https://www.fda.gov/news-events/fda-voices/looking-ahead-2022-fdas-center-devices-and-radiological-health-manages-sustained-increase-workload
5 Shuren, J., & Maisel, W. (2021, February 15). Reflections on a Record Year for Novel Device Innovation Despite COVID-19 Challenges. Retrieved from U.S. Food & Drug Administration: https://www.fda.gov/news-events/fda-voices/reflections-record-year-novel-device-innovation-despite-covid-19-challenges
6 Understanding the Breakthrough Devices Program. (2021, August 4). Retrieved from AdvaMed: Advanced Medical Technology Association: https://www.advamed.org/wp-content/uploads/2021/08/Ouided-Rouabhi_Breakthrough-Devices-Program-Presentation-.pdf
7 Safer Technologies Program for Medical Devices - Guidance for Industry and Food and Drug Administration Staff. (2021, January 6). Retrieved from U.S. Food & Drug Administration: https://www.fda.gov/media/130815/download
8 Medical Device De Novo Classification Process - A Rule by the Food & Drug Administration. (2021, October 5). Retrieved from Federal Register: https://www.federalregister.gov/documents/2021/10/05/2021-21677/medical-device-de-novo-classification-process
9 CMS Repeals MCIT/R&N Rule; Will Consider Other Coverage Pathways to Enhance Access to Innovative Medical Devices. (2021, November 12). Retrieved from Centers for Medicare & Medicaid Services: https://www.cms.gov/newsroom/press-releases/cms-repeals-mcitrn-rule-will-consider-other-coverage-pathways-enhance-access-innovative-medical
10 Electronic Submission Template for Medical Device 510(k) Submissions - Draft Guidance for Industry and Food and Drug Administration Staff. (2021, September 29). Retrieved from U.S. Food & Drug Administration: https://www.fda.gov/media/152429/download