 This is the last FDA Regulatory 101 installment of the year for our partnership with BioOhio. After events in Cincinnati and Columbus, the October 27th event will be held during the 2016 Heal Ohio Conference at the University of Akron. Former FDA Consumer Safety Officer Jake O'Donnell (25+ years of FDA experience) will be presenting and answering questions, in addition to other industry experts.
This is the last FDA Regulatory 101 installment of the year for our partnership with BioOhio. After events in Cincinnati and Columbus, the October 27th event will be held during the 2016 Heal Ohio Conference at the University of Akron. Former FDA Consumer Safety Officer Jake O'Donnell (25+ years of FDA experience) will be presenting and answering questions, in addition to other industry experts.
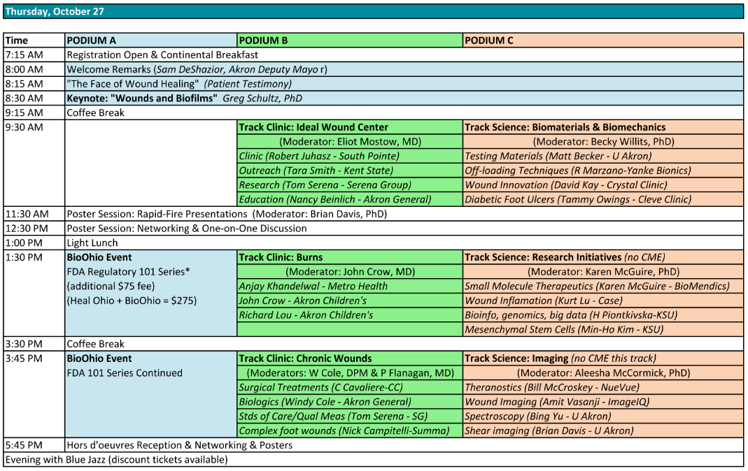
What's more, those who register for the 101 event are eligible for a discount to the conference. Keep reading to see the agenda and register to attend.
What will you learn and how about that agenda?
Apart from core regulatory principles, the event will cover the areas of quality systems, design assurance, product quality and post-market surveillance. A special combination products section will also be offered, presented by R&Q's Director of Regulatory Affairs, Nancy Morrison. Also as mentioned, Former FDA Consumer Safety Officer Jake O'Donnell will be presenting and answering questions. Come prepared and use his 25+ years of experience to your advantage! Here's how the agenda breaks out:
1:30 – 2:00 Regulatory Overview
2:00 – 2:30 Quality Systems Overview
2:30 – 3:00 Design Assurance Overview
3:00 – 3:30 Product Quality Overview
3:30 – 3:45 Coffee Break (Built-in to existing schedule; mandatory)
3:45 – 4:15 Post-Market Overview
4:15 – 4:45 Spotlight: Combination Products
4:45 – 5:30 Jake O'Donnell (former FDA) Q&A and Networking
Costs and registration
 Register for the event here. Here are the rates:
Register for the event here. Here are the rates:
Reg 101 Event + Heal Ohio Conference – $275
Reg 101 Event Only; BioOhio Member (companies w/ 10 employees or less) – $75
Reg 101 Event Only; BioOhio Member (companies w/ 11+ employees) – $125
Reg 101 Event Only; Non-members – $250
Reg 101 Event Only; BioOhio Student Members – $50
Anyone who registers for the FDA Regulatory 101 can take advantage of the discounted rate of $200 to attend the Heal Ohio Conference.
What next?
Easy: Register here. Startup company or not, a lifetime of regulatory advice in a day's worth of time and access to a former FDAer with so much experience is a tough opportunity to pass up. We hope you'll join us!



