Check out R&Q experts' European Union Medical Device Regulation (MDR) 2017/745 Health Check List below to determine the areas to focus your resources in the final few months before the May 26, 2020 MDR Date of Application. If you have questions, concerns, or need any help identifying the areas to prioritize, don't hesitate to reach out to your R&Q representative or contact us today.
1. Be realistic about where you are in the EU MDR transition process and use the resources available to you – we have many listed below!
a. Medical device manufacturers and Notified Bodies alike are being stretched thin, so the sooner you can identify what needs to be done the better – time is not on your side.
2. Know your Notified Bodies.
a. See a current list at the bottom of this article or conduct your own search here.
3. Do not underestimate the application process – it is very intense!
a. You are required to have level 6 CND codes if available, materials and primary function of devices to be identified. Note that CND codes are the product codes used under EU MDR, replacing GMDN as common nomenclature. You can reference the guidance on CND codes here.
b. Expect an average of three rounds of questions for companies with complex product lines.
4. Initiate scheduling for your initial Quality Management System (QMS) audits ASAP.
a. Audits are taking at least six months to be scheduled AFTER the application is accepted by the Notified Bodies and a contract is in place.
b. Anticipate much more stringent audits on clinical data, consequently you need to focus on closing any clinical evaluation gaps as well as your Post Market Clinical Follow-ups (PMCF).
5. Prioritize completing your Post Market Surveillance (PMS) plans, including PMCF plans, as they must be in place for all devices.
a. Although we always recommend full compliance, this is an area that we see many clients struggling due to the depth of new requirements and vague guidelines. Your R&Q representative can help you determine the next steps based on your unique needs.
b. Check out our free webinar on PMS Requirements of the EU MDR: Implementation Challenges and Solutions here for additional in-depth information on this topic.
6. Full EU MDR compliance is required if you have Class I devices that are not sterile, measuring or reusable surgical instruments. Full compliance is also required for custom Class III devices.
7. Vigilance reporting needs to follow EU MDR’s 15-day reporting timeline.
8. Registration was due but with the delay in the EUDAMED database to at least 2022, we are following MDD registration. Stay tuned for more guidance for Economic Operators/Manufacturers.
a. Check out our blog post on the EUDAMED delay here.
9. No new devices or significant changes in design or intended purpose.
a. For example, if you have a certificate for Class IIa devices in a certain family you cannot do a line extension after May 26, 2020 without an MDR certification.
10. The good news is that Class I reusable surgical instruments have been extended to May 26, 2024 for compliance, though they must be on an MDD Declaration of Conformity and meet the "no changes" statement above.
a. Be aware of the Implant Card guidance as it now requires a credit card size per ISO/IEC 7810.
b. Read our blog post about the EU MDR Class I device delay here.
11. Anticipate that new test results on legacy devices may result in a failure – so do not assume that doing testing for your legacy devices will get supporting evidence of safety/performance.
12. For United Kingdom distributed devices:
a. Brexit has happened but not in the “hard Brexit” format that we feared last year. Read our blog post on Brexit: Top Five Do's and Don'ts for Medical Device Manufacturers here.
b. It is time to find a new authorized representative now, since transition planning is in process and the current date is December 31, 2020 for the exit.
13. For Switzerland Notified Bodies and manufacturers:
a. Contingency plans may be required as Switzerland still has not completed the mutual recognition with the EU.
If you are struggling with the above items or having any other issues or concerns with your EU MDR transition, don't delay in contacting your R&Q representative - we are here to help!
Below are the current designated Notified Bodies for EU MDR pulled directly from the European Commission Internal Market, Industry, Entrepreneurship and SMEs Notified bodies Nando tool that can be found here.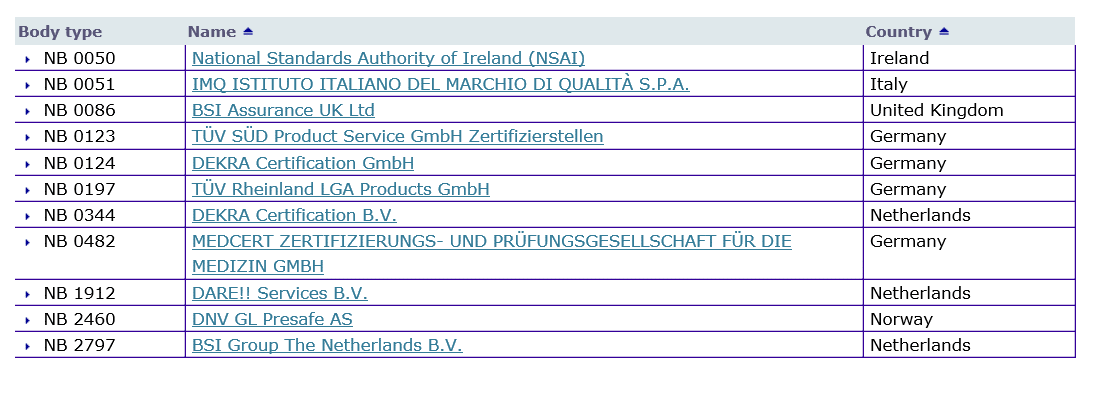
European Commission. Policies, information and services. Internal Market, Industry, Entrepreneurship and SMEs, Single Market and Standards, Tools and Databases, Notified bodies Nando. Accessed February 20, 2020. https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.notifiedbody&dir_id=34
R&Q Quicklinks:
– Interested in a career with R&Q? View our available positions.
– Are you following R&Q on LinkedIn? We believe you should be! We share hiring announcements, free webinars, events, news, and more.
– Same goes for R&Q's blog: we encourage you to subscribe!
– Need help with a project or have specific questions? Contact us.
About Regulatory & Quality Solutions (R&Q):

R&Q's mission is to improve people's lives by providing industry-leading regulatory and quality consulting and engineering for medical devices, IVDs, and combination products. We help companies bring safe and effective products to market… and keep them there. Our team of 200+ consultants and counting have served over 300 companies around the globe. Subscribe to our blog, view our service offerings, watch free webinars, and more at RQMplus.com.
The and means more.
For R&Q service inquiries, please use our Contact page.


