The FDA’s recently proposed rule on wound care products has sent ripples through MedTech. This rule, if finalized, could significantly impact the classification, labeling, and testing requirements for a wide range of wound care devices.
FDA is proposing to classify wound dressings (solid, gel, cream, or ointment dressings) and liquid wound washes containing antimicrobials as Class II or Class III medical devices. Wound dressings and washes containing antimicrobials of low or medium level antimicrobial resistance (AMR) concern would become Class II devices, whereas wound care devices containing antimicrobials of high level AMR concern would become Class III devices.
To date, the majority of these unclassified devices have required a 510(k) to bring them to the U.S. market. Based on the proposal, wound dressings and washes determined to be Class II devices would need to comply with new labeling requirements and special controls, including specific performance testing and risk assessments, within 6 months of the final rule. Those devices determined to be Class III devices would require a notice of intent to file a PMA within 90 days and a PMA would need to be submitted within 30 months of the final rule.
FDA published the proposed rule on November 30, 2023, and is expected to respond to comments and issue a final rule within the next 1-2 years. If the proposed rule is finalized, it is likely to have a significant impact on the classification, product labeling, and testing requirements for all wound care dressings and washes containing antimicrobials.
The RQM+ team will be hosting a live panel discussion on this topic “FDA’s Wound Care Shakeup: Ensuring Your Products Make the Cut”. Bring your questions and join the conversation as our team of FDA regulatory experts examine the proposed rule, providing valuable insights and actionable strategies to help you navigate what’s next for wound care devices. 👉 Register for free here.
Proposed Product Classifications
FDA is proposing to classify currently unclassified, pre-amendments wound dressings and liquid wound washes containing antimicrobials and/or other chemicals. At present, these products are regulated through the 510(k) pathway, but the proposed rule would replace the current product codes associated with wound care products containing antimicrobials as a preservative or protectant to reduce microbial growth within the dressing. If finalized, the new rule would apply to wound dressings and liquid wound washes categorized based on their physical state:
- Solid Wound Dressings containing antimicrobials and/or other chemicals: A solid wound dressing containing antimicrobials and/or other chemicals is used to cover and protect a wound, to absorb exudate, and to maintain appropriate moisture balance within the wound.
- Wound Dressings formulated as a Gel, Cream, or Ointment containing antimicrobials and/or other chemicals: A wound dressing formulated as a gel, cream, or ointment containing antimicrobials and/or other chemicals is used to maintain appropriate moisture balance within the wound.
- Liquid Wound Washes: A liquid wound wash is a water-based solution used to mechanically irrigate and physically remove debris from external wounds. It is also used to moisten solid wound dressings to maintain appropriate moisture balance within the dressing.
In terms of their regulation, devices containing antimicrobials as protectants or preservatives would be classified as either Class II or Class III medical devices depending on the level of concern around antimicrobial resistance (AMR). Wound dressings and liquid wound washes containing antimicrobials of low or medium level AMR concern would be designated as Class II medical devices, whereas wound dressings and liquid wound washes that contain “medically important” antimicrobials would be classified as Class III products. FDA intends to regulate these “medically important” devices more strictly, as they could contribute to the development and spread of microorganisms that are resistant to medically important antimicrobials, potentially further limiting clinicians’ therapeutic options.
| Level of risk | Example Chemicals | Classification | Pathway |
|---|---|---|---|
| Low level of risk antimicrobials | Parabens, Hypochlorous acid, Peroxide, PHMB (polyhexamethylene biguanide) | Class II | 510(k) |
| Medium level of risk antimicrobials | Silver, Zinc, Copper, Chlorhexidine, Benzalkonium Chloride | Class II | 510(k) |
| Medically important antimicrobials | Polymyxin B, Silver sulfadiazine, Bacitracin | Class III | PMA |
Intended Use, Indications for Use, Claims, and Labeling
With the proposed rule, the FDA is looking to make it clear that the antimicrobials and/or other chemicals in these wound dressings and liquid wound washes are not intended to treat an infection. Wound dressings and liquid wound washes within the scope of the proposed medical device classification rule are expected to only contain antimicrobials that support the use of the dressing or wash; either as a preservative or as a protectant of the product (e.g., to reduce microbial growth within a solid wound dressing while in use, to prevent or reduce contamination or deterioration of the product while in its packaging, etc.). If an antimicrobial plays a role other than as a preservative or protectant of the wound care product, then it would likely be considered a combination product and assessed based on its primary intended effect and mode of action.
FDA is also looking for manufacturers to be more explicit with regard to the intended use of their antimicrobial-containing wound care product. Historically, manufacturers of these wound dressings and liquid wound washes have stated that the intended use of their device is for “wound management” and/or to reduce a patient’s “risk of infection.”
With the proposed rule, manufacturers would be expected to no longer use general or misleading terms, and instead provide product labeling that explicitly states the intended use of the device (e.g., to cover and protect a wound, to absorb exudate, to maintain appropriate moisture balance, etc.). Manufacturers of wound care products already on the market may need to revise their product labeling to clarify the intended use of their device, as well as their claims.
Key claims that would not be available, would require specific clarifications, or would require evidence in the form of clinical data include:
- Use of the term “wound management”
- Use of the word “may” (e.g., “may reduce the risk of infection”)
- Claim that the device is a treatment or cure for wounds
- Claim that the device delivers antimicrobials to the wound
- Antimicrobial preservative claims (for a sterile, single-use product), and
- Preservative effectiveness claims (for wound dressings formulated as a gel, cream, or ointment, and liquid wound washes)
The following key claims are considered recognized benefits of the wound dressings and liquid wound washes according to the proposed rule:
- Maintain a moist wound healing environment
- Provide an effective barrier to environmental contaminants
- Reduce microbial growth within the dressing
- Extend the shelf life of nonsterile and/or multiuse wound dressings
By clarifying the intended use of the antimicrobial(s) and/or other chemicals in the product’s labeling and the Indications for Use statement, wound dressings and liquid wound washes would be regulated only as “devices” and not as combination products. If a manufacturer continues to or would like to make certain wound management claims, then they may be considered a combination product or drug and regulated as such.
Special Controls for Class II Devices
FDA has proposed a number of special controls as part of the new rule, which would require compliance within 6 months of the new rule being finalized. Depending on the wound care product, newly classified Class II wound dressings and liquid wound washes may be expected to provide additional performance testing and descriptive information, antimicrobial characterization and preservative effectiveness testing, an AMR risk assessment, additional biocompatibility evaluation, a risk management assessment for animal-derived materials and/or botanical extracts, updated labeling, shelf-life validation, and sterilization validation.
With respect to antimicrobial characterization and performance testing, FDA may expect to see performance data demonstrating that the antimicrobial has a purpose and is present in appropriate amounts to perform as intended. Testing would likely include establishment of the antimicrobial’s Minimum Effective Concentration (MEC) in the context of the final wound dressing, preservative effectiveness testing, and characterization of bioburden.
With regard to providing an AMR risk assessment, FDA will likely expect an evaluation and identification of any probable risks that the device could promote the development and spread of antimicrobial resistance. FDA would also likely expect to review specific risk management assessments for certain materials like animal-derived substances and botanical extracts.
Proposed Timelines
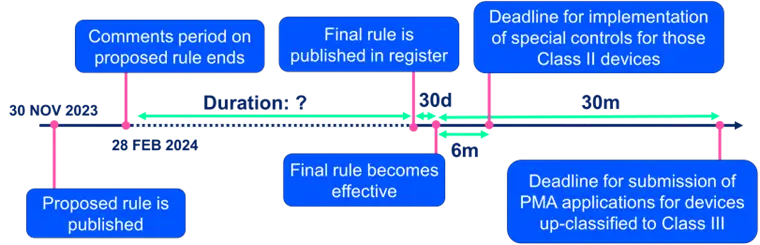
For wound care devices proposed to be classified into Class II that have prior 510(k) clearance, FDA will likely expect a new 510(k) to demonstrate compliance with newly established applicable special controls. The evidence of compliance would be expected within six months of the effective date of the final rule unless the manufacturer had already provided similar or applicable data to FDA in a prior 510(k) Premarket Notification. If the new rule is finalized, manufacturers of any newly classified Class II wound products would be expected to comply with all special controls and publicly state how the product meets those special controls as part of the product’s 510(k) Summary.
For wound care devices that are proposed to be classified into Class III, a notice of intent to file a PMA needs to be submitted within 90 days of the effective date of the order. FDA expects that manufacturers of Class III devices will submit a PMA within 30 months.
Conclusions
The FDA’s proposed rule supports greater regulation over medical devices that contain antimicrobials and other chemicals, even if they are only present to help preserve or protect the device, given their potential risk to patients and concern around increased AMR. Manufacturers of wound dressings and liquid wound washes that include antimicrobials or other chemicals should evaluate whether the new rule will impact their products.
It is important to determine whether a current marketing authorization is sufficient to meet the new rule, and if not, understand what action would be required to comply with the proposed special controls. Companies with questions around the proposed new rule or in need of support determining how the proposed new rule may impact their product’s current marketing claims should feel free to reach out to RQM+. We are here to help!
Don’t miss our RQM+ Live! online panel discussion on FDA’s Wound Care Shakeup. It could be your key to understanding the potential implications of this rule on your products and to prepare for the changes. Register for free here.
