By Chris A. Parr – Principal, CRO
As a follow up to “Recent developments in EU Horizontal Legislation” and “Medical Devices, IVDs and Other EU Laws” we will now demystify Europe, the EU and the EU single market so that manufacturers have an improved awareness of European countries and territories where they can achieve market access with the CE mark.
Europe and The European Union (EU)
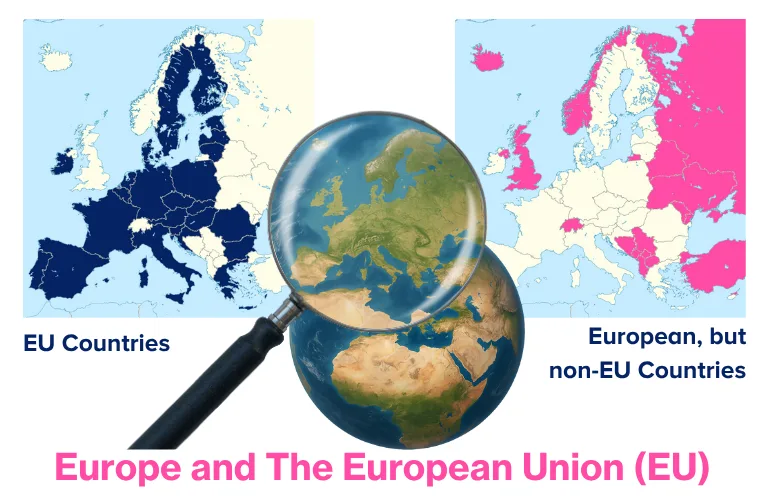
To set the scene, it is necessary to first define the terms “Europe” and the “European Union” (EU). Europe is one of the seven continents of the world and is a broad geographical term that includes all countries located on the European continent. Whereas the EU (see graphic below) is a political and economic union of 27 European countries1 that have agreed to work together more closely in areas like trade, law, security, and environmental policy. Not all European countries are members of the EU; 22 European countries are not members of the EU2.
The EU Single Market is one of the core foundations of the European Union. It allows for the free movement of four key things across all EU member states. The CE mark enables the free movement of medical devices and IVDs in the EU Single Market.
- Goods – Products can be traded across borders without tariffs or customs checks.
- Services – Businesses can offer services in any EU country.
- People – Citizens can live, work, study, or retire in any EU country.
- Capital – Money can move freely for investment, banking, or business.
European Free Trade Area (EFTA) and European Economic Area (EEA)
Now we have defined the EU and the EU Single Market, we will move on and cover the European Free Trade Association (EFTA) and the European Economic Area (EEA). EFTA is a regional trade organization and free trade area made up of four European countries. It was founded as an alternative to the then-European Economic Community (EEC), which later became the EU. EFTA promotes free trade and economic cooperation among its members and with other countries worldwide.
- Iceland
- Liechtenstein
- Norway
- Switzerland
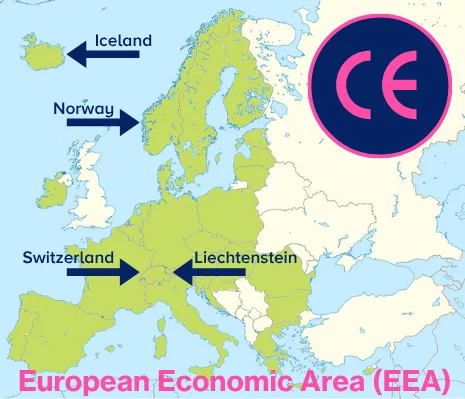
The European Economic Area (EEA) is a region that brings together the 27 EU member states and three of the four EFTA countries (Iceland, Liechtenstein, and Norway) into a single internal market. The CE mark enables the free movement of medical devices and IVDs in the EEA.
EU Outermost Regions
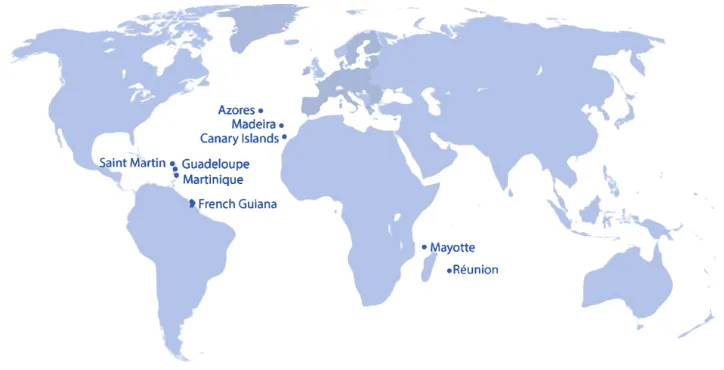
The EU Outermost Regions (ORs) are parts of the EU member states France, Portugal, and Spain that are geographically distant from mainland Europe but are fully part of the European Union (see details and map below). The name comes from Article 349 of the Treaty on the Functioning of the European Union (TFEU), which acknowledges that these regions:
- Are located far from continental Europe (often in the Caribbean, Indian Ocean, or Atlantic).
- Face permanent constraints such as:
- Remoteness
- Insularity
- Small size
- Difficult topography and climate

These regions are subject to EU law and benefit from special support due to their unique challenges, such as remoteness, insularity, and economic dependence. This means that medical devices and IVDs marketed in the Outermost Regions must have a valid CE mark, just like in mainland France, Spain, or Portugal.
Non-EU Countries with Customs Union Agreements
Turkey
Turkey is not part of the EU or EEA, instead it is part of a Customs Union with the EU that includes medical devices and IVDs. This means that medical devices and IVDs legally placed on the EU single market can also circulate in Turkey without additional customs duties or technical barriers, provided they comply with EU regulations. Devices must bear the CE mark to demonstrate conformity.
Andorra
Andorra, Monaco, and San Marino can be collectively described as European microstates. They are independent from the European Union (EU), but they each have special relationships with it.
Andorra is not an EU or EEA member but has a customs agreement with the EU. Andorra does not have its own medical device or IVD regulatory framework. It relies on EU regulations, meaning CE marking is required for medical devices and IVDs placed on the Andorran market. Devices must comply with MDR/IVDR if they are to be imported or distributed in Andorra.
Monaco
Monaco is not an EU or EEA member, but it is in a customs union with France. As a result, French and EU regulations apply, including those for medical devices and IVDs. CE marking is required for medical devices placed on the market in Monaco, as they must comply with MDR/IVDR through French oversight.
San Marino
San Marino is not an EU or EEA member, but it has close ties with Italy and a customs union with the EU. CE marking is required for medical devices and IVDs placed on the market in San Marino, as they must comply with MDR/IVDR through Italian oversight.
Switzerland
Switzerland had a Mutual Recognition Agreement (MRA) with the EU covering medical devices and IVDs. However, this agreement ceased to apply fully after the EU Medical Device Regulation (MDR) came into force in May 2021, due to the lack of an updated institutional framework agreement between the EU and Switzerland. Switzerland is not a member of the EU or EEA and now requires Swiss-specific regulatory compliance (MedDO4). Switzerland unilaterally recognizes CE-marked medical devices and IVDs that comply with MDR/IVDR.
United Kingdom
The United Kingdom of Great Britain and Northern Ireland officially left the European Union on January 31, 2020. Great Britain (England, Scotland Wales) is no longer an EU member and does not have a customs union agreement with the EU. In GB, manufacturers can use the UKCA mark or the CE mark which is recognised until 30 June 2030 at the latest5,6. In contrast, Northern Ireland remains in regulatory alignment with EU rules by virtue of the Windsor Framework Agreement. This means that it effectively remains part of the EU Single Market for goods only. Therefore, the CE mark is required for medical devices and IVDs placed on the NI market.
Summary
Medical device manufacturers must understand the distinctions between Europe, the EU, the EEA, EFTA, and the EU Outermost Regions because these geopolitical and regulatory groupings determine market access, regulatory compliance, and product certification requirements. In the EU and the EEA, medical devices and IVDs must comply with the MDR (EU) 2017/745 or IVDR (EU) 2017/746. This includes Iceland, Liechtenstein, and Norway that have adopted EU medical device and IVD regulations through the EEA Agreement. It also includes the EU Outermost Regions that are part of EU member states but located outside Europe. Compliance with the MDR/IVDR is also required in countries with customs union agreements with the EU and in Northern Ireland due to its unique geopolitical situation. Lastly, compliance with the MDR/IVDR is an option for Great Britain and Switzerland who continue to recognise the CE mark.
Related next steps
If you’re having difficulty navigating the EU regulatory landscape and struggling to comply with horizontal EU regulations, our experts specialise in helping manufacturers develop comprehensive regulatory strategies designed to ensure that medical devices and IVDs comply with all applicable EU legislation.
Contact us to discuss how we can support your efforts and stay tuned for more updates related to the European Medical Device landscape in our upcoming blog posts.
For further reading – Braving the Tariff Storm: Strategies for MedTech Companies
- https://european-union.europa.eu/principles-countries-history/eu-countries_en#header_countries_list
- https://www.worldatlas.com/geography/european-countries-that-are-not-members-of-the-european-union.html
- https://ec.europa.eu/regional_policy/policy/themes/outermost-regions_en
- https://www.fedlex.admin.ch/eli/cc/2020/552/en
- https://www.gov.uk/government/publications/implementation-of-the-future-regulation-of-medical-devices/implementation-of-the-future-regulations
- https://www.gov.uk/government/publications/implementation-of-the-future-regulation-of-medical-devices/statement-of-policy-intent-international-recognition-of-medical-devices